Abelmoschus manihot
Abelmoschus manihot
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Abelmoschus manihot
- Cat.No. Product Name CAS Number COA
-
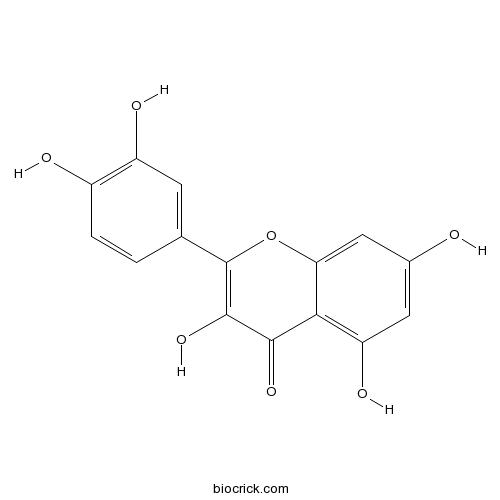
BCN6049
Quercetin117-39-5
Instructions
-
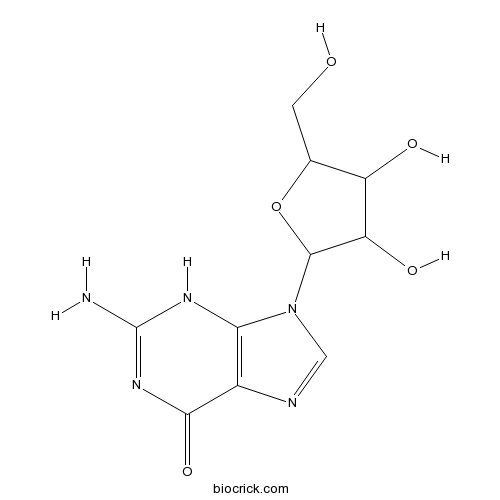
BCN2962
Guanosine118-00-3
Instructions
-
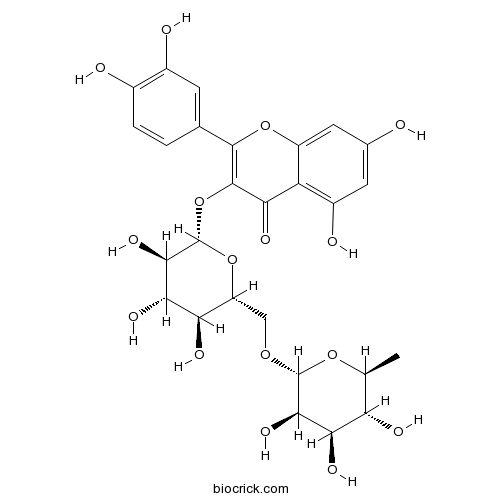
BCN1684
Rutin153-18-4
Instructions
-
BCN5160
Ampelopsin27200-12-0
Instructions

-
BCN5570
Hyperoside482-36-0
Instructions

-
BCN5692
Myricetin529-44-2
Instructions

-
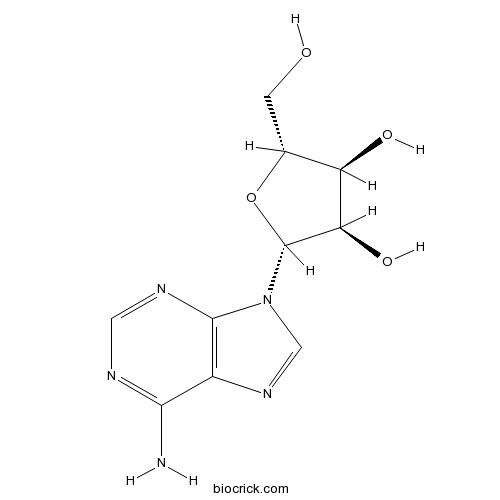
BCN5796
Adenosine58-61-7
Instructions
-
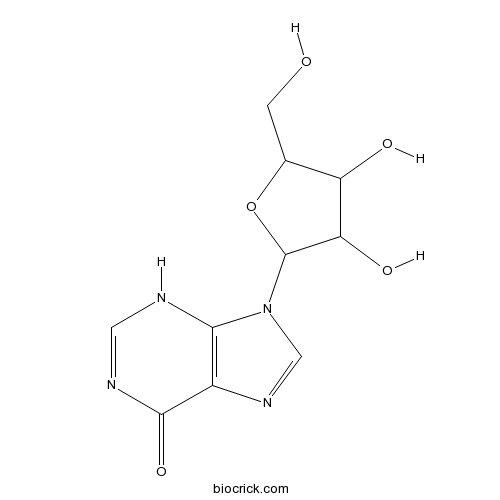
BCN3841
Inosine58-63-9
Instructions
-
BCC8260
Triacontanol 593-50-0
Instructions

Total flavones of Abelmoschus manihot improve diabetic nephropathy by inhibiting the iRhom2/TACE signalling pathway activity in rats.[Pubmed: 29221422]
Total flavones extracted from Abelmoschus manihot L. (Malvaceae) medic (TFA) have been proven clinically effective at improving renal inflammation and glomerular injury in chronic kidney disease (CKD).
[Effect of flavonoids from Abelmoschus manihot on proliferation, differentiation of 3T3-L1 preadipocyte and insulin resistance].[Pubmed: 28936849]
Abelmoschus manihot was rich in flavonoids, which has been reported the activity on protecting angiocarpy and improving renal function. This study aimed to explore the action mechanism of five flavonoids from A. manihot on how to ameliorating insulin resistance through the regulation of the glucose and expression of PPARγ, C/EBPα, SREBP-1, resistin, visfatin, adiponectin in 3T3-L1 adipocytes. After the 3T3-L1 preadipocytes were differentiated into mature adipocytes, insulin resistance model was built. Insulin resistance adipocytes were treated with 5, 100 μmol•L⁻¹ quercetin, isoquercitrin, hyperoside, quercitrin-3'-O-glucoside, gossypetin-8-O-β-glucoside. The glucose was indirectly determined by BCA kit. The mRNA expression levels of PPARγ, C/EBPα, SREBP-1, resistin, visfatin, adiponectin were detected by real-time quantitative PCR. Results showed that five flavonoids at 5 μmol•L⁻¹ could accelerate preadipocytes proliferation and inhibit that at 100 μmol•L⁻¹ Compared with the normal group, glucose uptake reduced significantly in model group (P<0.01). With the treatment of five flavonoids at 100 μmol•L⁻¹, glucose consumption increased significantly (P<0.01). The high expression of PPARγ, C/EBPα, adiponectin expression was significantly increased (P<0.01), and low expression of SREBP-1, resistin, visfatin after respective administration with five flavonoids at 100 μmol•L-1 promoted adipocyte differentiation. This study showed that, HY, JY, QT, QG, GG can control preadipocytes proliferation, promote adipocyte differentiation and regulate the expression of relative factors with lipid metabolism, such as PPARγ, C/EBPα, SREBP-1, adiponectin, resistin, visfatin, increasing glucose utilization and improving insulin resistance in 3T3-L1 adipocyte.
[Analysis and utilization value discussion of multiple chemical composition in different tissues of Abelmoschus manihot].[Pubmed: 28929656]
This research is to analyze the resourceful chemical composition in different tissues (root, stem, leaf and flower) of Abelmoschus manihot and evaluate their utilizing value. The flavonoids, soluble polysaccharides, cellulose, nucleosides and amino acids in the different tissues of A. manihot were determined by HPLC coupled with UV-Vis spectrophotpmetry, and UPLC-TQ/MS. The flowers are rich in the resourceful chemical compositions of flavonoids which mainly consist of hyperoside, isoquercitrin, cotton-8-O-glucuronide, myricetin, quercetin-3'-O-glucoside, rutin and quercetin. The total content of these flavonoids is 25.450 mg•g-1 in the flowers, while they are trace in the other tissues.Different tissues of A. manihot are rich in soluble polysaccharides and celluloses and the stems have the highest content(19.76%) of soluble polysaccharides, while the roots have the highest content (29.88%) of cellulose. Total of 21 amino acids and 9 nucleosides were detected in this plant, and the flowers have the highest content of amino acids(4.737 mg•g⁻¹), while the leaves have the highest content of nucleosides (1.474 mg•g⁻¹). A. manihot is rich in the resourceful chemical compositions, and its constituents and contents are various in different tissues of this plant.The results provided a scientific basis for the utilization and industrial development of A. manihot plants.
Characterization and immunomodulatory activity of polysaccharides from the stems and leaves of Abelmoschus manihot and a sulfated derivative.[Pubmed: 28860057]
Abelmoschus manihot (Linn.) Medicus is a traditional herbal medicine whose flowers, stems and leaves exhibit widely pharmacological activities. However, only the flowers have long been used as medicine while the stems and leaves were mainly discarded and burned, which undoubtedly caused enormous waste of these resources and serious environment pollution. Many researches have indicated that bioactivities of polysaccharides were significantly improved after sulfation. The aim of this study was to investigate the characterization and immunomodulatory activity of polysaccharides from stems and leaves of A. manihot and a sulfated derivative.
Dynamic changes of flavonoids in Abelmoschus manihot different organs at different growth periods by UPLC-MS/MS.[Pubmed: 28558340]
Abelmoschus manihot (Linn.) Medicus has been clinically used to treat chronic kidney disease, oral ulcers, burns, and dysmenorrhea in China for many centuries. The major pharmacologically-active components of A. manihot are flavonoids. In this study, a rapid and highly sensitive UPLC-MS/MS analysis method was established and successfully applied to the simultaneous determination of five major flavonoids (rutin, hyperoside, isoquercitrin, quercetin, and myricetin) in different parts of A. manihot harvested at ten growth periods. Under the optimized chromatographic conditions, good separation for five target components was obtained on an Acquity UPLC BEH C18 column within 18min. The total contents of the five investigated flavonoids in A. manihot roots, stems, leaves and flowers ranged from 2.86 to 123.7μg/g, 46.39 to 141.0μg/g, 929.4 to 3096μg/g, and 10,150 to 19,390μg/g, respectively, indicating that the total flavonoids in the four parts could be mainly arranged in a decreasing order as flower>leaf>stem>root. The peak of total flavonoids in flowers and leaves appeared at G8 and G9, respectively. These results will be helpful for the determination of the suitable harvest time of A. manihot and the improvement of the utility value of the disused parts.
Total Flavonoid Extract from Abelmoschus manihot (L.) Medic Flowers Attenuates d-Galactose-Induced Oxidative Stress in Mouse Liver Through the Nrf2 Pathway.[Pubmed: 28472605]
Abelmoschus manihot (L.) Medic is an edible hibiscus that is rich in flavonoids, and its use as Chinese herbal medicine for the treatment of diseases and health maintenance dates back to ancient times. The chemical compositions of total flavonoid of A. manihot (L.) Medic flower extract (TFAE) were identified and determined by high performance liquid chromatography (HPLC). The effects of TFAE on antioxidative activities in a d-galactose (d-gal)-induced mouse model and Nrf2-mediated antioxidant responses were evaluated. Male Kunming mice were randomly divided into normal control group, d-gal aging model group, d-gal+ascorbic acid group that served as a positive control, and d-gal+TFAE (40, 80, and 160 mg TFAE/kg) group. After 42 days, the antioxidant effects of these treatments were determined by biochemical studies, Western blotting, quantitative real-time polymerase chain reaction, and histological analysis. The results showed that the groups administered TFAE exhibited significant elevation in liver activities of antioxidant enzymes, including catalase (CAT), glutathione peroxidase (GPx), superoxide dismutase (SOD), and total antioxidant capacity (T-AOC), and decreased malondialdehyde (MDA) production in a dose-dependent manner compared with the d-gal-induced model group. Expression of Nrf2 and its target antioxidants (HO-1 and NQO1) was manifestly increased by TFAE treatment. TFAE also increased mRNA expression of GPx, SOD, and CAT and decreased tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β). Furthermore, the microstructure of livers in TFAE-administered mice was obviously improved as compared with the d-gal model group. These results suggest that TFAE protects mice against d-gal-induced oxidative stress, and the effect is related to the activation of Nrf2 signaling.


