Ajuga ciliata
Ajuga ciliata
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Ajuga ciliata
- Cat.No. Product Name CAS Number COA
-
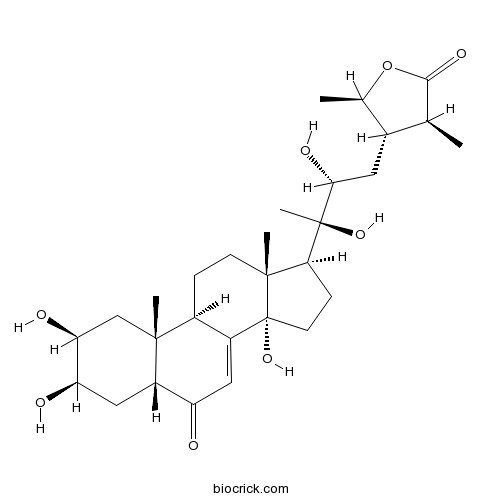
BCN5416
Cyasterone17086-76-9
Instructions
-
BCN4256
8-O-Acetylharpagide6926-14-3
Instructions

neo-Clerodane diterpenes from Ajuga ciliata Bunge and their neuroprotective activities.[Pubmed: 21807075]
Three new (1-3) and three known (4-6) neo-clerodane diterpenes have been isolated from the whole plants of Ajuga ciliata Bunge. The structures of the new compounds were elucidated as (12S)-1β,6α,19-triacetoxy-18-chloro-4α,12-dihydroxy-neo-clerod-13-en-15,16-olide (1), (12S,2'S)-12,19-diacetoxy-18-chloro-4α,6α-dihydroxy-1β-(2-methylbutanoyloxy)-neo-clerod-13-en-15,16-olide (2), and (12S)-6α,18,19-triacetoxy-4α,12-dihydroxy-1β-tigloyloxy-neo-clerod-13-en-15,16-olide (3), on the basis of spectroscopic data analysis. All the diterpenes were evaluated for the neuroprotective effects against MPP(+)-induced neuronal cell death in dopaminergic neuroblastoma SH-SY5Y cells and compounds 2-5 exhibited moderate neuroprotective effects.
Bioactive neo-clerodane diterpenoids from the whole plants of Ajuga ciliata Bunge.[Pubmed: 21682262]
Ten new neo-clerodane diterpenes, ajugaciliatins A-J (1-5, 8-12), along with 17 known analogues (6, 7, 13-27) were isolated from the whole plants of Ajuga ciliata Bunge. Their structures were elucidated by spectroscopic data analysis (IR, ESIMS, HRESIMS, 1D and 2D NMR), and the configuration of 1 was confirmed by X-ray crystallography. All of the compounds were assessed for neuroprotective effects against MPP(+)-induced neuronal cell death in dopaminergic neuroblastoma SH-SY5Y cells. Compounds 2, 6, 7, 9, 10, 15-17, 19, and 20 exhibited moderate neuroprotective effects.


