Ajuga decumbens
Ajuga decumbens
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Ajuga decumbens
- Cat.No. Product Name CAS Number COA
-
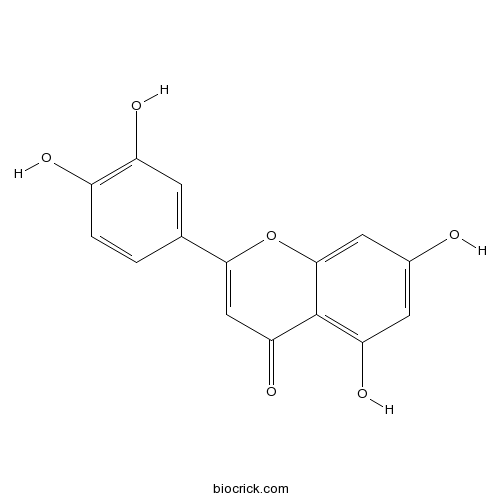
BCN5600
Luteolin491-70-3
Instructions
Neo-clerodane diterpenes and phytoecdysteroids from Ajuga decumbens Thunb. and evaluation of their effects on cytotoxic, superoxide anion generation and elastase release in vitro.[Pubmed: 29894737]
Five novel compounds, including four neoclerodane diterpenoids, named ajugacumbins KN (1-4) along with a phytoecdysterone, named ajugacetalsterone E (5), were isolated from the whole herbs of Ajuga decumbens (Labiatae). Their structures were elucidated on the basis of detailed spectroscopic analysis including IR, HRESIMS, CD, 1D and 2D NMR spectroscopic experiments. Compounds 1-5 were evaluated for their cytotoxic activities and the effects on superoxide anion generation and elastase release in FMLP/CB-induced human neutrophils.
A New Succinate Derivative from Ajuga decumbens.[Pubmed: 27396203]
A new succinate derivative, ethyl (5-formylfuran-2-yl)methyl succinate (1), along with three known compounds (2-4) have been isolated from the whole plants of Ajuga decumbens Thunb. Their structures were elucidated by extensive spectroscopic (1D and 2D NMR) and HR-ESI-MS data analysis, and literature values. Compound 1 was isolated as a new succinate derivative, and compounds 2 and 3 were for the first time separated from A. decumbens.
[Effects of the extracts from Ajuga decumbens on anti-fatigue in mice].[Pubmed: 29931885]
None
Structure elucidation and biological activity of two new trichothecenes from an endophyte, Myrothecium roridum.[Pubmed: 24909753]
Worldwide, many different grains are infected by various fungi that may produce trichothecene mycotoxins. Fungi that produce trichothecenes, as well as the trichothecenes themselves, are potential problems for public health. On the other hand, trichothecenes possess multiple biological activities. Reduced toxicity may result in their applications in the pharmaceutical field. Two new trichothecenes along with seven known trichothecenes were isolated from an endophyte of the herb plant Ajuga decumbens. Their structures were deduced from 1D and 2D NMR data. The results of MTT assays revealed that new trichothecene 2',3'-epoxymyrothecine A, 1, and myrothecine A, 3, exhibited much lower toxicity compared to other trichothecenes. New trichothecene 2',3'-epoxymyrothecine A, 1, could induce phosphorylation of JNK (c-Jun N-terminal protein kinase) protein and the PARP (poly ADP-ribose polymerase) cleavage, and eventually induce apoptosis in cancer cells. These results point out the possibility for application of trichothecenes as chemotherapeutic agent.
Classification and identification of metal-accumulating plant species by cluster analysis.[Pubmed: 24888623]
Identification and classification of metal-accumulating plant species is essential for phytoextraction. Cluster analysis is used for classifying individuals based on measured characteristics. In this study, classification of plant species for metal accumulation was conducted using cluster analysis based on a practical survey. Forty plant samples belonging to 21 species were collected from an ancient silver-mining site. Five groups such as hyperaccumulator, potential hyperaccumulator, accumulator, potential accumulator, and normal accumulating plant were graded. For Cd accumulation, the ancient silver-mining ecotype of Sedum alfredii was treated as a Cd hyperaccumulator, and the others were normal Cd-accumulating plants. For Zn accumulation, S. alfredii was considered as a potential Zn hyperaccumulator, Conyza canadensis and Artemisia lavandulaefolia were Zn accumulators, and the others were normal Zn-accumulating plants. For Pb accumulation, S. alfredii and Elatostema lineolatum were potential Pb hyperaccumulators, Rubus hunanensis, Ajuga decumbens, and Erigeron annuus were Pb accumulators, C. canadensis and A. lavandulaefolia were potential Pb accumulators, and the others were normal Pb-accumulating plants. Plant species with the potential for phytoextraction were identified such as S. alfredii for Cd and Zn, C. canadensis and A. lavandulaefolia for Zn and Pb, and E. lineolatum, R. hunanensis, A. decumbens, and E. annuus for Pb. Cluster analysis is effective in the classification of plant species for metal accumulation and identification of potential species for phytoextraction.


