Akebia trifoliata
Akebia trifoliata
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Akebia trifoliata
- Cat.No. Product Name CAS Number COA
-
BCN2787
Calceolarioside B105471-98-5
Instructions

-
BCN5159
alpha-Hederin27013-91-8
Instructions

-
BCN5171
Geniposidic acid27741-01-1
Instructions

-
BCN5979
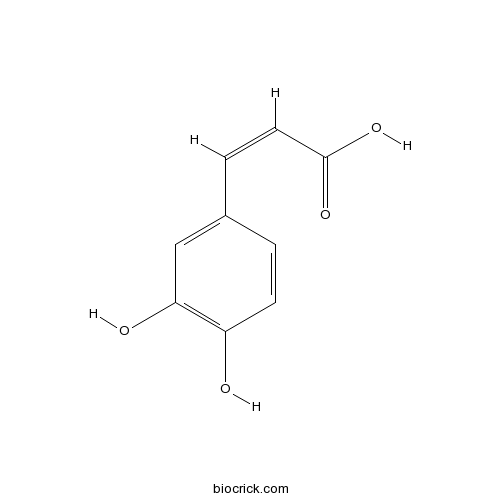
Caffeic acid331-39-5
Instructions
-
BCN5608
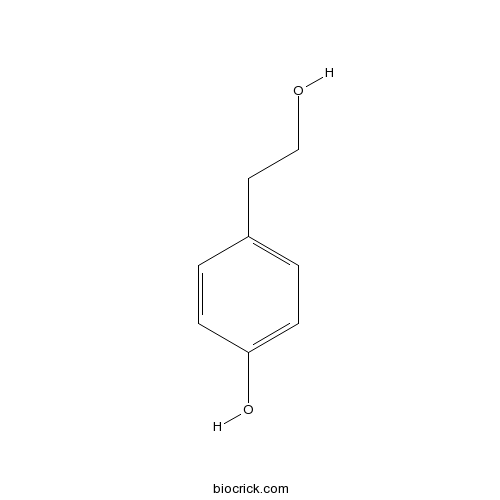
2-(4-Hydroxyphenyl)ethanol501-94-0
Instructions
-
BCN4376
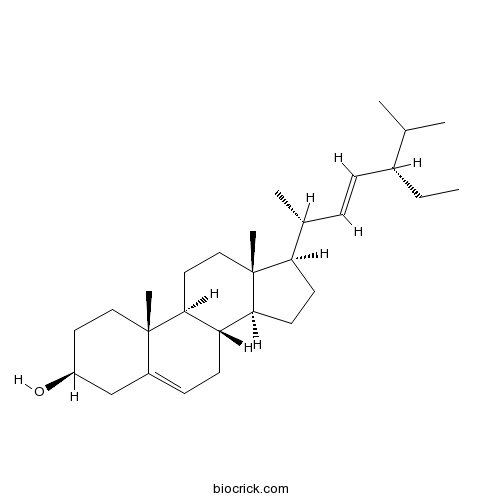
Stigmasterol83-48-7
Instructions
Two New Pentacyclic Triterpene Saponins from the Leaves of Akebia trifoliata.[Pubmed: 27455226]
Two new pentacyclic triterpene saponins, named akebiaoside K (1) and akebiaoside N (2), were isolated from the leaves of Akebia trifoliata, together with five known triterpenoids 3-7. They were all isolated from the leaves of A. trifoliata for the first time. Their structures were established by spectral and chemical means. Triterpenes 5 and 7 were found to show moderate in vitro cytotoxicity against human tumor A549, HeLa and HepG2 cell lines, with IC50 values ranging from 0.023 to 0.038 mM. Triterpenes 5-7 were further revealed to show significant in vitro α-glucosidase inhibitory activity with IC50 values from 0.040 to 0.220 mM, making them more potent than the reference compound acarbose (IC50 0.409 mM). Meanwhile, no obvious inhibitory effects were observed for the isolated triterpene saponins 1-4 in both bioactivity assays.
[Study on ecological environment and accompanying plants' community characteristics study of wild Panax japonicus in Enshi].[Pubmed: 28891605]
The paper is aimed to study the distribution, population density, soil conditions and community characteristics of accompanying plants' in Enshi sub-regional different areas, with a typical habitats investigation method. The results showed that the wild Panax japonicus mainly distributed in moist places under the forests, by streams, or secondary forests of high grass, within east longitude 29°-30°, north latitude 108°-110°and about 1 000-15 00 meters above sea level. The soils were mainly tide soil and humus with yellow-brown soil, yellow soil and red soil, and the humus thickness was5-30 centimeter, pH 6.0-6.8, the moisture content of 16.8%-24.2%, soil bulk density of 1.39-2.12. Its geographical vegetation types were mainly evergreen coniferous forest, evergreen-deciduous mixture broad leaved forest and evergreen coniferous forest mixed deciduous broad-leaved forest, including three levels community structure of arbors, shrubs and herbaceous; Its accompanying plants reached 86 families, 118 genera, 134 species of seed plants, the arbors included 15 families, 21 genera, 26 species and the dominant species community mainly Pinaceae such as Pinus massoniana, P. tabuliformis, P. henryi and Taxodiaceae such as Cunninghamia lanceolata, Cryptomeria fortunei etc. The shrubs included 39 families, 54 genera, 62 species with the dominant species such as Camellia oleifera, Kalopanax septemlobus, Akebia trifoliata, Trachycarpusfortunei, Rhamnus globosa, Smilax corbularia and so on. The herbaceous included 32 families, 43 genera, 46 species, and Ferns such as the black-footed Dryopteris, Dryopteris crassirhizom, Coniogramme affinis, Polystichum tripteron, Adiantum pedatum, Lunathyrium acrostichoides, Woodsia ilvensis and Woodwardia japonica were dominant species. The cover layer covered a large number of lichens and mosses. The wild P. japonicus can be found among the P. massoniana, P. tabuliformis, P. henryi, lichens and mosses. These may indicate that the wild P. japonicusin Enshi requires higher demands on the ecological environment, its accompanying plants are mainly the tree layer-shrub layer-herb layer, and vertical structure is obvious. The study provides a basis for domestication and conservation of P. japonicus resources.
Native fruit tree genetic resources in Japan.[Pubmed: 27069393]
The diversity of climate, from subarctic to subtropical, and the complex geological history of Japan have produced a rich biodiversity. The flora includes several hundred species of native woody plants with edible fleshy fruits or nuts. People have eaten them from prehistoric times until about a half century ago. In Hokkaidō and the Ryūkyū Islands nut species had an important role in the diet, but fleshy fruits were also eaten until recently. Only Castanea crenata and a few minor species became domesticated as edible fruit trees in pre-modern times. Recently, Vitis coignetiae, Lonicera caerulea, Akebia quinata, Akebia trifoliata, Stauntonia hexaphylla, and Actinidia arguta have entered small-scale cultivation. The conservation of the germplasm of many of these native species, both in situ and ex situ, is precarious.
Phylogenomic and structural analyses of 18 complete plastomes across nearly all families of early-diverging eudicots, including an angiosperm-wide analysis of IR gene content evolution.[Pubmed: 26724406]
The grade of early-diverging eudicots includes five major lineages: Ranunculales, Trochodendrales, Buxales, Proteales and Sabiaceae. To examine the evolution of plastome structure in early-diverging eudicots, we determined the complete plastome sequences of eight previously unsequenced early-diverging eudicot taxa, Pachysandra terminalis (Buxaceae), Meliosma aff. cuneifolia (Sabiaceae), Sabia yunnanensis (Sabiaceae), Epimedium sagittatum (Berberidaceae), Euptelea pleiosperma (Eupteleaceae), Akebia trifoliata (Lardizabalaceae), Stephania japonica (Menispermaceae) and Papaver somniferum (Papaveraceae), and compared them to previously published plastomes of the early-diverging eudicots Buxus, Tetracentron, Trochodendron, Nelumbo, Platanus, Nandina, Megaleranthis, Ranunculus, Mahonia and Macadamia. All of the newly sequenced plastomes share the same 79 protein-coding genes, 4 rRNA genes, and 30 tRNA genes, except for that of Epimedium, in which infA is pseudogenized and clpP is highly divergent and possibly a pseudogene. The boundaries of the plastid Inverted Repeat (IR) were found to vary significantly across early-diverging eudicots; IRs ranged from 24.3 to 36.4kb in length and contained from 18 to 33 genes. Based on gene content, the IR was classified into six types, with shifts among types characterized by high levels of homoplasy. Reconstruction of ancestral IR gene content suggested that 18 genes were likely present in the IR region of the ancestor of eudicots. Maximum likelihood phylogenetic analysis of a 79-gene, 97-taxon data set that included all available early-diverging eudicots and representative sampling of remaining angiosperm diversity largely agreed with previous estimates of early-diverging eudicot relationships, but resolved Trochodendrales rather than Buxales as sister to Gunneridae, albeit with relatively weak bootstrap support, conflicting with what has been found for these three clades in most previous analyses. In addition, Proteales was resolved as sister to Sabiaceae with the highest support (bootstrap >90%) yet observed in plastome-scale phylogenetic analyses.
The effect of deamidation on the structural, functional, and rheological properties of glutelin prepared from Akebia trifoliata var. australis seed.[Pubmed: 25704689]
The characteristics of glutelin samples from Akebia trifoliata var. australis seeds (AG) that had been deamidated by malic acid (MDAG) and by citric acid (CDAG) were investigated. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis showed high-molecular-weight subunits that were degraded into smaller fragments, and FTIR indicated a decrease in the number of β-sheet groups and an increase in the amount of β-turns in the deamidated samples. These results could be caused by the cleaving of partial disulfide bonds to form new sulfhydryl groups during deamidation. Citric acid was found to be more effective at deamidation and hydrolysis, resulting in a higher solubility and emulsifying activity for CDAG, and MDAG also exhibited some improvement in terms of surface hydrophobicity and emulsion ability. Rheology showed that the gelation point for deamidated samples was increased, and the gel network was strengthened. The amounts of essential amino acids that were well-preserved and the improved solubility, emulsification, and rheology properties of AG after acid-heating deamidation show that this technique can be useful for treating other plant-based food ingredients in the future.
Nortriterpene Saponins from Akebia trifoliate.[Pubmed: 25632461]
Three new nortriterpene saponins, 2α,3β,20α-trihydroxy-30-norolean-12-en-28-oic acid O-β-D-xylopyranosyl-(1-->3)-α-L-rhamnopyranosyl-(1-->4)-β-D- glucopyranosyl-(1-->6)-β-D-glucopyranosyl ester (3), 2α,3β,20α-trihydroxy-30-norolean-12-en-28-oic acid O-α-L-rhamnopyranosyl-(1-->4)-β-D- glucopyranosyl-(1-->6)-β-D-glucopyranosyl ester (4), and 2α,3β,23-trihydroxy-30-noroleana-12,19-dien-28-oic acid O-β-D-xylopyranosyl-(1-->3)-α-L- rhamnopyranosyl-(1-->4)-β-D-glucopyranosyl-(1-->6)-β-D-glucopyranosyl ester (6), were isolated from the methanol extract of the pericarps of Akebia trifoliata Koidzumi (Lardizabalaceae), together with five known nortriterpene saponins and three phenolic glycosides. Their structures were elucidated by spectroscopic analyses and acid hydrolysis.
[Caffeoylquinic acid derivatives from stems of Akebia trifoliata].[Pubmed: 25566654]
To study the chemical constituents from Akebia trifoliata stems.
[Accumulation dynamic of triterpenoid saponins in Akebia trifoliata stem].[Pubmed: 25566644]
In order to understand the accumulation dynamic of triterpenoid saponins in Akebia trifoliata stem to determine the suitable harvesting time and the growth age of stem.
Antibacterial oleanane-type triterpenoids from pericarps of Akebia trifoliata.[Pubmed: 25172756]
Three new oleanane triterpenoids, 2α,3β,29-trihydroxyolean-12-en-28-oic acid (1), 2α,3β-dihydroxy-23-oxo-olean-12-en-28-oic acid (2) and 2α,3β,21β,22α-tetrahydroxyolean-12-en-28,29-dioic acid (3), and ten known ones, maslinic acid (4), arjunolic acid (5), oleanolic acid (6), 3-epi-oleanolic acid (7), stachlic acid A (8), serratagenic acid (9), gypsogenic acid (10), 2α,3β-dihydroxyol-ean-13(18)-en-28-oic acid (11), mesembryanthemoidigenic acid (12) and 12α-hydroxy-δ-lactone (13), were isolated from the pericarps of Akebia trifoliata, a new valued fruit crop in China. Their structures were elucidated on the basis of extensive spectroscopic analysis. Compounds 8, 10, 11 and 13 were isolated for the first time from the genus Akebia. All the compounds were tested for their antimicrobial activity against five bacterial strains. Compounds 4, 6 and 11 showed significant antibacterial activity toward all the assayed microorganisms with MIC values ranging from 0.9 to 15.6μg/mL, which were close or even more potent than the reference compound Kanamycin (MIC values ranging from 1.9 to 3.9μg/mL).


