Ancistrocladus tectorius
Ancistrocladus tectorius
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Ancistrocladus tectorius
- Cat.No. Product Name CAS Number COA
-
BCN5597
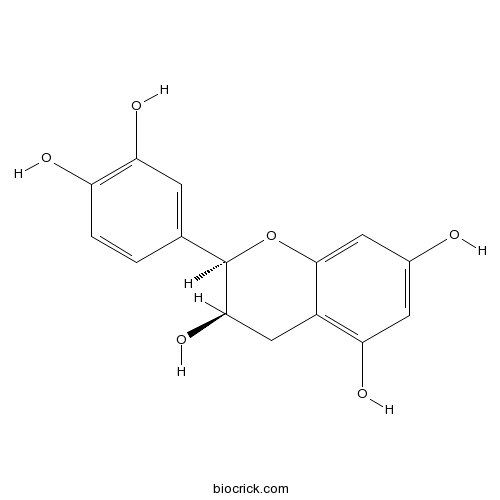
Epicatechin490-46-0
Instructions
-
BCN2707
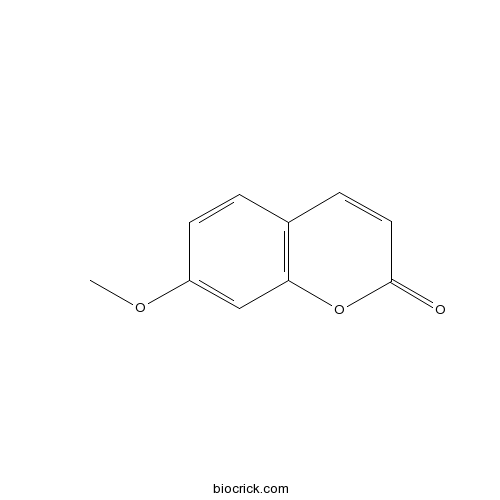
7-Methoxycoumarin531-59-9
Instructions
Ancistrocyclinones A and B, unprecedented pentacyclic N,C-coupled naphthylisoquinoline alkaloids, from the Chinese liana Ancistrocladus tectorius.[Pubmed: 29443340]
Two unique pentacyclic N,C-coupled naphthylisoquinolines, the ancistrocyclinones A (5) and B (6), were discovered in the Chinese liana Ancistrocladus tectorius. Furthermore, six known, likewise N,C-coupled alkaloids, viz., ancistrocladinium A (7a) and its mono- and bisphenolic analogs 8a and 9a were isolated, along with their atropo-diastereomers 7b, 8b, and 9b. The stereostructures of 5 and 6 were determined by HRESIMS, 1D and 2D NMR, oxidative degradation, and ECD calculations. The pentacyclic ancistrocyclinones A (5) and B (6) are structurally similar to berberine alkaloids - yet arising from a most different biosynthetic pathway: they are apparently formed by N,C-coupling of their polyketide-derived molecular halves, followed by oxidative cyclo-condensation. Biomimetic conversion of the co-occurring 4'-O-demethylancistrocladinium A (8a) to ancistrocyclinone A (5) via a quinoid intermediate supported the postulated pathway. Ancistrocyclinone A (5) was found to significantly inhibit the viability of drug-sensitive human leukemia (CCRF-CEM) and multidrug-resistant tumor cells (CEM/ADR5000) with comparable efficacies.
Antileukemic ancistrobenomine B and related 5,1'-coupled naphthylisoquinoline alkaloids from the Chinese liana Ancistrocladus tectorius.[Pubmed: 28688886]
A striking feature of the metabolite pattern of the Southeast Asian liana Ancistrocladus tectorius (Ancistrocladaceae) is the predominance of 5,1'-coupled naphthylisoquinoline alkaloids. About 20 alkaloids of this coupling type have so far been discovered in this plant species. Here, we report on the isolation of four new 5,1'-linked naphthylisoquinolines from the twigs and stems of A. tectorius. Two of them, the ancistrobenomines B (5) and C (6), belong to the very rare group of alkaloids with a fully dehydrogenated isoquinoline portion. Likewise unusual for naphthylisoquinoline alkaloids is the presence of a hydroxymethylene group at C-3. Within the large class of meanwhile ca. 180 such natural products, this structural peculiarity had so far been known only from two other representatives isolated from the Malaysian species A. benomensis, and from one single naphthalene-devoid 3-hydroxymethyleneisoquinoline from A. tectorius. Seven further 5,1'-linked alkaloids, previously isolated from related Asian and African Ancistrocladus species, have now been identified for the first time in A. tectorius. Their structural elucidation was achieved by spectroscopic analysis including HRESIMS, 1D and 2D NMR, and by chemical (oxidative degradation) and chiroptical (electronic circular dichroism) methods. Ancistrobenomine B (5) exhibited moderate effects against Plasmodium falciparum and Trypanosoma brucei rhodesiense in vitro, and it was found to display strong cytotoxic activities against drug-sensitive acute lymphoblastic CCRF-CEM leukemia cells and their multidrug-resistant subline, CEM/ADR5000.
Ancistectorine D, a naphthylisoquinoline alkaloid with antiprotozoal and antileukemic activities, and further 5,8'- and 7,1'-linked metabolites from the Chinese liana Ancistrocladus tectorius.[Pubmed: 27646602]
None
Five novel naphthylisoquinoline alkaloids with growth inhibitory activities against human leukemia cells HL-60, K562 and U937 from stems and leaves of Ancistrocladus tectorius.[Pubmed: 24076380]
Two new 7,6'-coupled naphthylisoquinolines, namely ancistrotectorines A (1) and B (2), two new 5,3'-coupled naphthylisoquinolines, namely ancistrotectorines C (3) and D (4), and one new 7,8-coupled naphthylisoquinoline, namely ancistrotectorine E (5), together with 9 known naphthylisoquinoline alkaloids, hamatine (6), ancistrobertsonine B (7), ancistrocladinine (8), hamatinine (9), ancistrotanzanine A (10), ancistrotanzanine B (11), ancistrotectoriline B (12), 7-epi-ancistrobrevine D (13), and ancistrotectorine (14), were isolated from the 70% EtOH extract of Ancistrocladus tectorius. Their structures were elucidated based on the extensive analysis of spectroscopic data (1D, 2D NMR and MS). Compound 5 exhibited inhibitory activities against HL-60, K562 and U937 cell lines with IC50 values of 1.70, 4.18 and 2.56 μM respectively.
Highly selective antiplasmodial naphthylisoquinoline alkaloids from Ancistrocladus tectorius.[Pubmed: 22459968]
Naphthylisoquinoline alkaloids, named ancistectorine A1, N-methylancistectorine A1, ancistectorine A2, 5-epi-ancistectorine A2, ancistectorine A3, ancistectorine B1, and ancistectorine C1, have been isolated from twigs of the Chinese plant Ancistrocladus tectorius. The structural elucidation succeeded by chemical, spectroscopic, and chiroptical methods. Three of these compounds exhibited excellent, and specific, antiplasmodial activities, comparable with that of the as yet most active representative, dioncophylline C. Moreover, the antitumoral activities of two of the main alkaloids in this species was tested.
Two new naphthylisoquinoline alkaloids from stems and leaves of Ancistrocladus tectorius.[Pubmed: 20552520]
Two new alkaloids, 4'-O-demethylhamatine (1) and ancistrotectoriline C (2), were isolated from the stems and leaves of Ancistrocladus tectorius. These two compounds represent one 5,1'-coupled and one 7,6'-coupled naphthylisoquinoline alkaloid, respectively. Their structures were elucidated by 1D- and 2D-NMR spectra and other spectroscopic studies.
Effect of Thai plant extracts on P-glycoprotein function and viability in paclitaxel-resistant HepG2 cells.[Pubmed: 20460821]
The effects of ethanol extracts from Thai plants on P-glycoprotein (P-gp) function and cell viability were examined using paclitaxel-resistant HepG2 (PR-HepG2) cells. KP018 from Ellipeiopsis cherrevensis and AT80 from Ancistrocladus tectorius increased both rhodamine 123, a typical P-gp substrate, and [(3)H]paclitaxel uptake in PR-HepG2 cells. However, some extracts such as MT80 from Microcos tomentosa increased rhodamine 123, but not [(3)H]paclitaxel, uptake, while MM80 from Micromelum minutum increased only [(3)H]paclitaxel uptake. Thus, the effects of extracts of Thai plants on rhodamine 123 uptake were not necessarily the same as those on [(3)H]paclitaxel uptake. Purified compounds such as bergapten did not affect the uptake of either substrate. KP018, AT80, and MM80 increased [(3)H]paclitaxel uptake and decreased the cell viability in a concentration-dependent manner. Among these extracts, KP018 showed the most potent cytotoxicity. The cytotoxic potency of KP018 on PR-HepG2 cells was similar to that on wild-type HepG2 cells, and was not potentiated by verapamil. At concentrations resulting in no cytotoxicity, AT80 and MM80 potentiated paclitaxel-induced cytotoxicity in PR-HepG2 cells. These results indicate that K018 may be a useful source to search for a new anticancer drug, while AT80 and MM80 may be useful as modulators of P-gp-mediated multidrug resistance in cancer cells.
Shuangancistrotectorines A-E, dimeric naphthylisoquinoline alkaloids with three chiral biaryl axes from the Chinese plant Ancistrocladus tectorius.[Pubmed: 20235249]
Five novel dimeric naphthylisoquinoline alkaloids, shuangancistrotectorines A (3 a), B (3 b), C (4), D (5 a), and E (5 b), have been isolated from the twigs of the Chinese plant Ancistrocladus tectorius. Their absolute stereostructures were determined by spectroscopic and chiroptical methods in combination with quantum chemical CD calculations. In contrast to all other known dimeric naphthylisoquinoline alkaloids, in which the central binaphthalene axis is 6',6''-coupled and thus not rotationally hindered, the dimers described here are linked via the sterically more hindered 3',3''- or 1',1''-positions of the naphthalene units. They are thus the first such dimers-and even the very first natural products at all-that have three consecutive stereogenic axes. Hence, including the stereogenic centers, they have up to seven stereogenic units in total. Some of the compounds, in particular shuangancistrotectorines A, B, and D (3 a, 3 b, and 5 a) exhibit very good, and specific, antiplasmodial activities.
Antimicrobial screening of plants used for traditional medicine in the state of Perak, Peninsular Malaysia.[Pubmed: 14693223]
Seventy-two extracts (methanol) obtained from the leaves, barks, and roots of 50 plant species used in the traditional medicine of Perak, Peninsular Malaysia, have been screened for antibacterial and antifungal activities. Peristrophe tinctoria, Polyalthia lateriflora, Knema malayana, Solanum torvum, Celosia argentea, Eclipta prostrata, Ancistrocladus tectorius, Dillenia suffruticosa, Piper stylosum and Rafflesia hasseltii displayed the broadest spectrum of activity.
Four new naphthylisoquinoline alkaloids from Ancistrocladus tectorius.[Pubmed: 11076558]
Four new naphthylisoquinoline alkaloids, ancistrotectoriline A (1), ancistrotectoriline B (2), 6-O-methyl-4'-O-demethylancistrocladine (3), and 6-O-methyl-4'-O-demethylhamatine (4), were isolated from the stems and leaves of Ancistrocladus tectorius, collected from Hainan Province, Southern China. Their structures were elucidated using MS and NMR methods.


