Asterella angusta
Asterella angusta
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Asterella angusta
- Cat.No. Product Name CAS Number COA
-
BCN1178
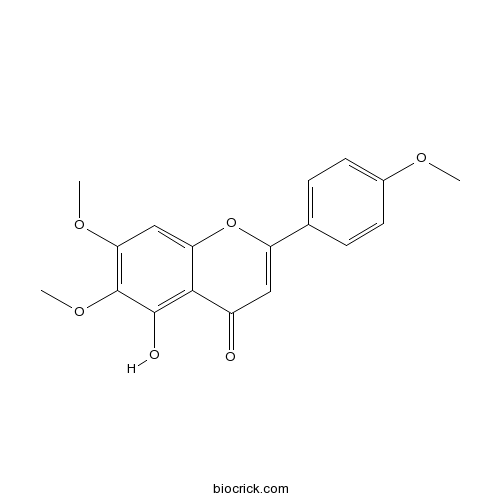
Salvigenin19103-54-9
Instructions
Dihydroptychantol A, a macrocyclic bisbibenzyl derivative, induces autophagy and following apoptosis associated with p53 pathway in human osteosarcoma U2OS cells.[Pubmed: 21185854]
Dihydroptychantol A (DHA), a novel macrocyclic bisbibenzyl compound extracted from liverwort Asterella angusta, has antifungal and multi-drug resistance reversal properties. Here, the chemically synthesized DHA was employed to test its anti-cancer activities in human osteosarcoma U2OS cells. Our results demonstrated that DHA induced autophagy followed by apoptotic cell death accompanied with G₂/M-phase cell cycle arrest in U2OS cells. DHA-induced autophagy was morphologically characterized by the formation of double membrane-bound autophagic vacuoles recognizable at the ultrastructural level. DHA also increased the levels of LC3-II, a marker of autophagy. Surprisingly, DHA-mediated apoptotic cell death was potentiated by the autophagy inhibitor 3-methyladenine, suggesting that autophagy may play a protective role that impedes the eventual cell death. Furthermore, p53 was shown to be involved in DHA-mediated autophagy and apoptosis. In this connection, DHA increased nuclear expression of p53, induced p53 phosphorylation, and upregulated p53 target gene p21(Waf1/Cip1). In contrast, cytoplasmic p53 was reduced by DHA, which contributed to the stimulation of autophagy. In relation to the cell cycle, DHA decreased the expression of cyclin B₁, a cyclin required for progression through the G₂/M phase. Taken together, DHA induces G₂/M-phase cell cycle arrest and apoptosis in U2OS cells. DHA-induced apoptosis was preceded by the induction of protective autophagy. DHA-mediated autophagy and apoptosis are associated with the cytoplasmic and nuclear functions of p53.
Synthesis and multidrug resistance reversal activity of dihydroptychantol A and its novel derivatives.[Pubmed: 19540127]
The macrocyclic bisbibenzyl dihydroptychantol A (DHA), previously isolated from Asterella angusta, was synthesized and showed significant multidrug resistance (MDR) reverting activity in chemoresistant cancer cells. In an attempt to discover more potent MDR reversal agents for efficient cancer chemotherapy, DHA derivatives with thiazole rings (19-22) were synthesized, and their cytotoxicities and MDR reversal activities were evaluated in adriamycin-resistant K562/A02, vincristine-resistant KB/VCR and in their parental cells by MTT assays. In response to treatment with each compound, the K562 cell line was the most sensitive, and the vincristine-resistant KB/VCR cell line was the most resistant. Marked decreases in K562 and K562/A02 cell viability were detectable after treatment with the synthesized derivatives of DHA, while less inhibitory effects on cell growth were observed in chemical-resistant KB/VCR and KB cells. Moreover, among the tested compounds, the intermediate 17 and the analogues 19, 20, and 21 showed potent MDR reversal activities and increased vincristine cytotoxicity in KB/VCR cells, with the reversal fold ranges from 10.54 to 13.81 (10microM), which is 3.2-4.3-fold stronger than the natural product DHA.
Reversal of p-glycoprotein-mediated multidrug resistance by macrocyclic bisbibenzyl derivatives in adriamycin-resistant human myelogenous leukemia (K562/A02) cells.[Pubmed: 18938236]
Macrocyclic bisbibenzyls, a class of characteristic natural molecules derived from liverworts, have diverse biological significances. Dihydroptychantol A (DHA) was identified to be an antifungal active macrocyclic bisbibenzyl from liverwort Asterella angusta. In an attempt to understand other biological activities of this compound, the chemical synthesized DHA and its analogues (compounds 1-3) were employed to test this possibility by using adriamycin-resistant K562/A02 cells. Among the tested compounds (1-4), DHA showed the strongest potency to increase adriamycin cytotoxicity toward K562/A02 cells by MTT assays and its reversal fold is 8.18 (20 microM). Mechanisms of DHA on p-glycoprotein (P-gp)-mediated multidrug resistance (MDR) were further investigated. Based on the flow cytometry, we detected the significant increase of adriamycin and rhodamine123 accumulation in K562/A02 cells exposed to various concentrations of DHA, meanwhile, notable decrease of rhodamine123 efflux was also observed, which revealed DHA caused a decline of P-gp activity. Furthermore, P-gp expression was analyzed by the flow cytometry and RT-PCR. Dose-dependent reduction of P-gp expression was measured in K562/A02 cells pretreated with DHA for 24h. No such results were found in parental K562 cells. These results demonstrated DHA reversed effectively MDR by blocking the drugs to be pumped out via inhibiting P-gp function and expression pathway.
Rapid screening for bisbibenzyls in bryophyte crude extracts using liquid chromatography/tandem mass spectrometry.[Pubmed: 17610245]
A simple and rapid qualitative liquid chromatography-diode-array detection/tandem mass spectrometry (LC-DAD/MS/MS) method was developed and validated for screening bisbibenzyl compounds in bryophyte crude extracts at sub-ppm levels. After simple extraction with ethanol and analyte concentration with diethyl ether, the extracts were subjected to LC-DAD/MS/MS analysis. The overall instrument turnaround time was 50 min to obtain baseline separation of bisbibenzyl isomers in bryophytes. MS full scan, MS/MS precursor ion scan and MS/MS product ion scan modes were used for the screening. The bisbibenzyl standards studied gave limits of detection (LODs) at or below 10 ng/mL. The results also indicated that the method had acceptable precision to be used on a day-to-day basis for qualitative identification. The bisbibenzyl types, i.e. one biphenyl ether bond (A-type), two biphenyl ether bonds (B-type), one biphenyl ether and one biphenyl bond (C-type), or other biphenyl types can be differentiated by their ESI-MS/MS product profiles, and the number of alkoxyl substituents can also be identified. The linkage sites of biphenyl and biphenyl ether bonds cannot be identified for an unknown bisbibenzyl solely from its mass spectra. This system was used to support three screening assays of bryophytes including Marchantia polymorpha L., Ptagiochasm intermedium L. and Asterella angusta, which were collected from different places in China. From them, 7/12, 8/5 and 8/9 confirmed/unconfirmed bisbibenzyls were identified, respectively, based on their MS/MS data, UV spectra and the retention behavior. The screening method considerably reduced the time and the cost for the qualitative analyses, and the structure-fragmentation-UV relationships will facilitate the high-throughput screening (HTS) of bisbibenzyl compounds in bryophytes. It is also intended as a simple and convenient way for the determination of other structural families of natural products.
Antifungal dibenzofuran bis(bibenzyl)s from the liverwort Asterella angusta.[Pubmed: 17570447]
Bioactivity-guided separation of an antifungal extract from the liverwort Asterella angusta afforded four bis(bibenzyl)s, asterelin A (1), asterelin B (2), 11-O-demethyl marchantin I (3), and dihydroptychantol A (4), together with six known ones. Their structures were established by extensive spectroscopic analysis (1D and 2D-NMR, MS), and that of 2 was confirmed by X-ray crystallographic diffraction analysis. Compounds 1 and 2 are the first examples of dibenzofuran bis(bibenzyl)s. The antifungal activity of the isolated bis(bibenzyl)s against the common clinical pathogenic fungus Candida albicans was evaluated using both the thin-layer chromatography bioautographic assay and the broth microdilution method. They showed moderate antifungal activities with minimal inhibitory concentration (MIC) values ranging from 16 microg/ml to 512 microg/ml.
Allelopathic effects of Lantana camara Linn. on spore germination of Asterella angusta Steph.--a liverwort.[Pubmed: 11906121]
Extract from root, stem and leaf of L. camara proved inhibitory for germination of the spores of A. angusta. Leaf extract was found to exhibit maximum allelopathic potentiality followed by stem and root extract and may be interpreted to be the result of phytotoxic substances which are possibly synthesized in the leaf and translocated to other organs.


