Catunaregam spinosa
Catunaregam spinosa
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Catunaregam spinosa
- Cat.No. Product Name CAS Number COA
-
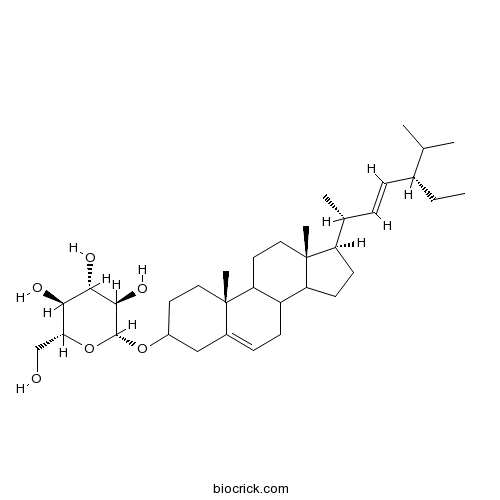
BCN4865
Stigmasterol glucoside19716-26-8
Instructions
-
BCN4546
4-Hydroxybenzoic acid99-96-7
Instructions

Green chemical approach towards the synthesis of SnO2 NPs in argument with photocatalytic degradation of diazo dye and its kinetic studies.[Pubmed: 27450298]
There are variety of effluents are dumped or directly discarded into atmosphere due to drastic industrialization which leads to damages in living organisms. To prevent many type of environmental defects our research group focused to synthesis material which degrades toxic substance like dyes with the help of ecofriendly synthesis. We have synthesized Tin oxide nanoparticles (SnO2 NPs) using aqueous extract of Catunaregam spinosa (C. spinosa) root barks. Bio-inspired synthesized SnO2 NPs were monitored by analytical characterization which inferred that SnO2 NPs resulted in shape of spherical, with size average of 47±2nm. Further bio-green synthesized SnO2 NPs were subjected to degrade toxic Congo red dye, which results in higher percentage of degradation with the K value of 0.9212 which obeys pseudo-first order reaction kinetics. This report said to be novel due to null report on SnO2 NPs synthesized from C. spinosa root bark aqueous extract which also stated to be simplest, cheaper and non-toxic while compare to other methods. Further to identify the metabolites which is present in the aqueous extract were identified through Gas Chromatography and Mass Spectrometry with methanol as a solvent results that 7-hydroxy-6-methoxy-2H-1-benzopyran-2-one contains higher area percentage of 67.47 with the retention time (RT) of 18.660.
Catunarosides I-L, four new triterpenoid saponins from the stem bark of Catunaregam spinosa.[Pubmed: 25765061]
Four new triterpenoid saponins, Catunaroside I [3-O-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-arjunic acid 28-O-β-D-glucopyranoside] (1), Catunaroside J [3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-arjunic acid 28-O-β-D-glucopyranoside] (2), Catunaroside K [3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-tormentic acid] (3), and Catunaroside L [3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-pomolic acid] (4), and two known triterpenoid saponin Arjunetoside (5) and Randiasaponin VII (6), were isolated from the stem bark of Catunaregam spinosa. Their structures were elucidated on the basis of their spectral data and chemical evidence.
Ethno-veterinary control of bovine dermatophilosis and ticks in Zhombe, Njelele and Shamrock resettlement in Zimbabwe.[Pubmed: 23054800]
A structured questionnaire survey was conducted to determine the ethno-veterinary practices and other control methods used by smallholder farmers for the management of bovine dermatophilosis and ticks. A total of 153 farmers were interviewed from Njelele, Zhombe communal and Shamrock resettlement areas. Crop production contributed most to livelihoods (83.2 %) while livestock contributed 9.0 %. Over 90 % of the respondents had attended school up to primary level, with 11.4 % undergoing animal health and husbandry training. Treatment of livestock diseases was practised by 96 % of the farmers, and 49.7 % of these farmers used ethno-veterinary medicines. Across the study sites, dermatophilosis was controlled using the following plants: Cissus quadrangularis (59.7 %), Catunaregam spinosa (10.5 %), Pterocarpus angolensis (10.5 %), Kalanchoe lanceolata (5.3 %), Aloe chabaudii (3.5 %), Cassia abbreviata (1.8 %), Dichrostachys cinerea (1.8 %), Urginea sanguinea (1.8 %), Ximenia caffra (1.8 %) and a plant locally called umfanawembila (1.8 %). Carica papaya and two plants, locally called mugimbura and umdungudungu, were used for tick control, and these were reported once from Njelele communal. Other control methods, besides plants or conventional drugs, were used by 28 % of the farmers for the treatment of dermatophilosis and ticks. Some farmers (14.4 %) claimed that ethno-veterinary medicines performed better than conventional drugs. The study revealed that farmers used ethno-veterinary medical practices for the treatment of dermatophilosis but rarely for tick control.
Triterpenoid saponins with antifeedant activities from stem bark of Catunaregam spinosa (Rubiaceae) against Plutella xylostella (Plutellidae).[Pubmed: 21861991]
Seven triterpenoid saponins, including four new compounds, catunarosides A-D (1-4), and three known compounds, swartziatrioside (5), aralia-saponin V (6), araliasaponin IV (7) were isolated from the stem bark of Catunaregam spinosa, a Chinese mangrove associate. Their structures were elucidated on the basis of their spectral data and hydrolysis experiments. The antifeedant activities of compounds 1-7 against Plutella xylostella were also evaluated.
Minor compounds from the stem bark of Chinese mangrove associate Catunaregam spinosa.[Pubmed: 18717492]
A new dihydroisocoumarin 3-(2-hydroxypropyl)-8-hydroxyl-3,4-dihydroisocoumarin (1) and a new iridoid 3-deoxyartselaenin C (5), together with six known compounds scopoletin (2), scoparone (3), morindolide (4), pinoresinol (6), medioresinol (7), and secoisolariciresinol (8) were isolated from the stem bark of Chinese mangrove associate Catunaregam spinosa Tirveng. The structures of 1 and 5 were determined by extensive spectroscopic analysis, including 1D and 2D NMR data. All compounds except 2 were obtained from C. spinosa for the first time. And it was also the first time lignans were obtained from Catunaregam sp.


