Clerodendrum trichotomum
Clerodendrum trichotomum
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Clerodendrum trichotomum
- Cat.No. Product Name CAS Number COA
-
BCN6148
Taraxerol127-22-0
Instructions
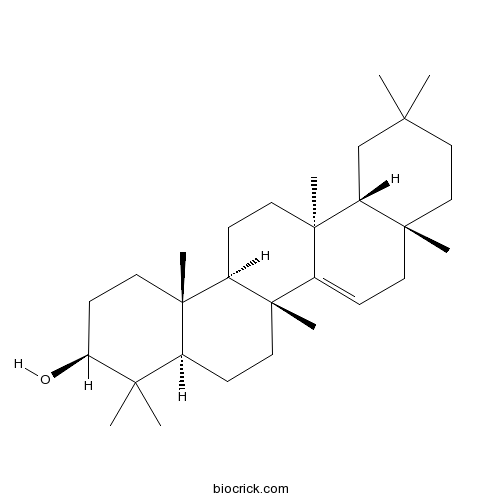
-
BCN1673
Phytol150-86-7
Instructions
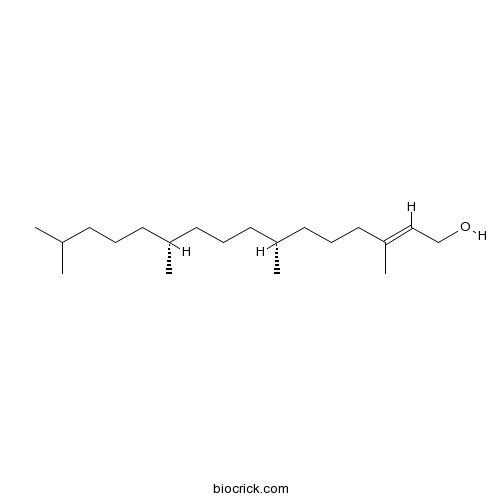
-
BCN5658
Apigenin520-36-5
Instructions

-
BCN5725
Lupeol545-47-1
Instructions

-
BCN4136
Acteoside61276-17-3
Instructions

Abietane Diterpenoids from the Roots of Clerodendrum trichotomum and Their Nitric Oxide Inhibitory Activities.[Pubmed: 29924604]
None
[Chemical constituents of Clerodendrum trichotomum Leaves].[Pubmed: 25857158]
To investigate the chemical constituents of the leaves of Clerodendrum trichotomum.
Abietane diterpenoids from Clerodendrum trichotomum and correction of NMR data of Villosin C and B.[Pubmed: 25230490]
Nine abietane diterpenoids (1-9) were isolated from the stems of Clerodendrum trichotomum Thunb. and identified by spectroscopic methods. Furthermore, corrected NMR data is provided for Villosin C (1) and B (2) whose absolute configurations were elucidated from circular dichroism (CD) data. All isolates were tested for cytotoxicity against four cancer cell lines (A549, HepG-2, MCF-7 and 4T1). Compounds 1, 2, 3, 4 and 8 were found to have remarkable cytotoxic effects with IC50 values ranging from 8.79 to 35.46 microM.
Poor correlation between the removal or deposition of pollen grains and frequency of pollinator contact with sex organs.[Pubmed: 23928839]
Pollinators deposit pollen grains on stigmas and remove pollen grains from anthers. The mechanics of these transfers can now be quantified with the use of high-speed video. We videoed hawkmoths, carpenter bees, and swallowtail butterflies pollinating Clerodendrum trichotomum. The number of grains deposited on stigmas did not vary significantly with the number of times pollinators contacted stigmas. In contrast, pollen removal from the anthers increased significantly with the number of contacts to anthers. Pollen removal varied among the three types of pollinators. Also, the three types carried pollen on different parts of their bodies. In hawkmoths and carpenter bees, a large number of contacted body part with anthers differed significantly from the body part that attached a large number of pollen grains. Our results indicate that a large number of contacts by pollinators does not increase either the male or female reproductive success of plants compared to a small number of contacts during a visit.
New cytotoxic steroids from the leaves of Clerodendrum trichotomum.[Pubmed: 23583601]
A phytochemical investigation of the leaves of Clerodendrum trichotomum led to the isolation of five new (2-6) and two known (1 and 7) steroids, whose structures and relative configurations were elucidated by comprehensive spectroscopic analysis and by comparison of their NMR data with those of related compounds. Steroids 2 and 5 exhibited moderate cytotoxicity in vitro against HeLa cell line.
Rearranged abietane diterpenoids from the roots of Clerodendrum trichotomum and their cytotoxicities against human tumor cells.[Pubmed: 23462587]
The roots of the medicinal ornamental plant Clerodendrum trichotomum yielded a series of rearranged abietane diterpenoids, including three 17(15→16)-abeo-abietane (1-3) and three 17(15→16),18(4→3)-diabeo-abietane (4-6) derivatives. Their structures were elucidated by means of spectroscopic methods. The absolute configuration of (10R,16R)-12,16-epoxy-11,14,17-trihydroxy-6-methoxy-17(15→16)-abieta-5,8,11,13-tetraene-7-one (1) was deduced by a combination of single crystal X-ray diffraction analysis and the observed Cotton effects in its circular dichroism (CD) spectrum. All isolates were tested for their cytotoxicities against five human cancer cell lines (BGC-823, Huh-7, KB, KE-97, and Jurkat). Among them, compounds 4, 6, 9, 10, 12, and 14, each possessing a common 17(15→16),18(4→3)-diabeo-abietane framework, were found to have remarkable cytotoxic effects with IC50 values ranging from 0.83 to 50.99 μM.


