Elephantopus tomentosus
Elephantopus tomentosus
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Elephantopus tomentosus
- Cat.No. Product Name CAS Number COA
-
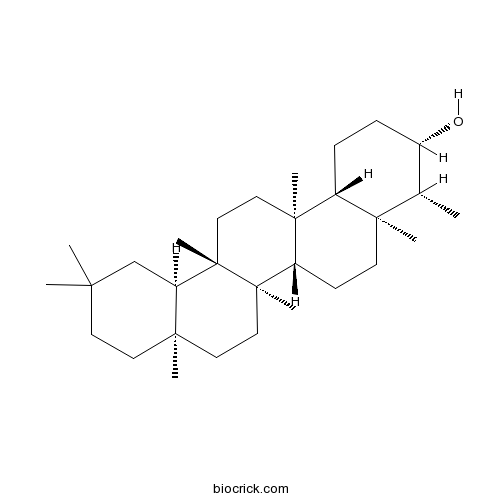
BCN1104
Epifriedelanol16844-71-6
Instructions
-
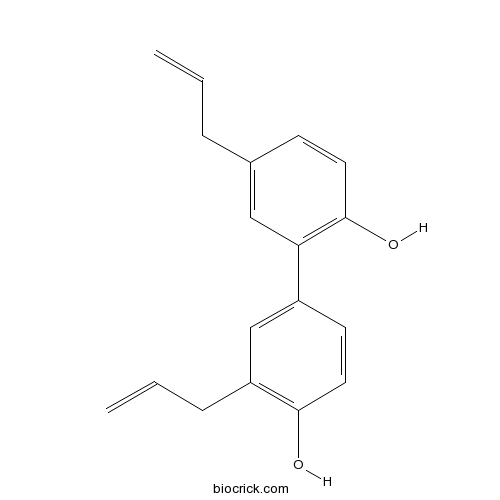
BCN1001
Honokiol35354-74-6
Instructions
Tomenphantadenine, an unprecedented germacranolide-adenine hybrid heterodimer from the medicinal plant Elephantopus tomentosus L.[Pubmed: 29197542]
An unusual adenine-substituted germacrane sesquiterpene lactone, tomenphantadenine (1), has been isolated from the whole plant of Elephantopus tomentosus L. The structure of this compound was established by comprehensive spectroscopic analysis including high resolution (HR) ESI-MS, 1D and 2D nuclear magnetic resonance (NMR) spectroscopic data. This compound features novel hybrid pattern of germacrane sesquiterpene with adenine through C-N linkage, and a possible biosynthetic pathway for it was proposed. Compound 1 showed potent antibacterial activity against the gram-positive Staphylococcus aureus and weak acetylcholinesterase (AChE) inhibitory activity.
[Chemical constituents from Elephantopus tomentosus].[Pubmed: 24010290]
To study the chemical constituents of Elephantopus tomentosus.
A new sesquiterpene lactone from Elephantopus tomentosus.[Pubmed: 22582752]
A new sesquiterpene lactone, named tomenphantopin H (1), together with two known germacranolides, 2β-methoxy-2-deethoxy-8-O-deacylphantomolin-8-O-tiglinate (2) and 2-deethoxy-2-hydroxyphantomolin (3), was isolated from the whole plant of Elephantopus tomentosus Linn. The new compound was completely elucidated using a combination of 1D and 2D NMR techniques (COSY, HMQC, and HMBC) and HR-ESI-MS analyses. All compounds exhibited antibacterial activity.
Anti-inflammatory and analgesic effects of Elephantopus tomentosus ethanolic extract.[Pubmed: 20633503]
Elephantopus tomentosus is widely used in Asia, especially in Malaysia, for the treatment of pain and inflammation. In the present study, the analgesic and anti-inflammatory effects of a 95% ethanol extract of E. tomentosus were investigated in different experimental models. In the anti-inflammation study, 1000 mg/kg of extract significantly reduced carrageenan-induced hind paw edema (p < 0.05) and inhibited abdominal permeability compared with control (p < 0.01). The analgesic activity was assayed in several experimental models in mice: (1) hot plate, (2) tail flick, (3) writhing test; and rats: carrageenan-induced hyperalgesia pain threshold test. However, at the doses tested, no significant activity was found in the hot plate test and the tail flick test. E. tomentosus ethanol extract at 1000 mg/kg significantly (p < 0.05) increased hyperalgesia pain threshold and inhibited writhing activity. The results suggest that E. tomentosus ethanol extract at 1000 mg/kg dose is effective in anti-inflammatory and non-steroidal anti-inflammatory drug type anti-nociception activities.
Antitumor agents. 190. Absolute stereochemistry of the cytotoxic germacranolides, tomenphantins A and B, from Elephantopus tomentosus.[Pubmed: 10075766]
The structures and absolute stereochemistries of tomenphantins A (1) and B (2), cytotoxic germacranolides isolated from Elephantopus tomentosus, are reported herein. 1H and 13C NMR spectroscopic data, chemical transformation, and single-crystal X-ray analysis were used in these determinations.


