Garcinia cowa
Garcinia cowa
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Garcinia cowa
- Cat.No. Product Name CAS Number COA
-
BCN6283
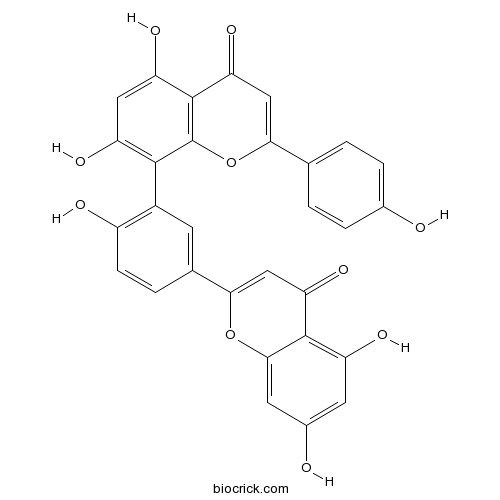
Amentoflavone1617-53-4
Instructions
Cytotoxic Properties and Complete Nuclear Magnetic Resonance Assignment of Isolated Xanthones from the Root of Garcinia cowa Roxb.[Pubmed: 27041859]
To isolate compounds from the roots of Garcinia cowa and to evaluated their cytotoxic activity against breast (MCF-7), prostate (DU-145), and lung (H-460) cell lines.
Large Scale Screening of Ethnomedicinal Plants for Identification of Potential Antibacterial Compounds.[Pubmed: 26985889]
The global burden of bacterial infections is very high and has been exacerbated by increasing resistance to multiple antibiotics. Antibiotic resistance leads to failed treatment of infections, which can ultimately lead to death. To overcome antibiotic resistance, it is necessary to identify new antibacterial agents. In this study, a total of 662 plant extracts (diverse parts) from 222 plant species (82 families, 177 genera) were screened for antibacterial activity using the agar cup plate method. The aqueous and methanolic extracts were prepared from diverse plant parts and screened against eight bacterial (two Gram-positive and six Gram-negative) species, most of which are involved in common infections with multiple antibiotic resistance. The methanolic extracts of several plants were shown to have zones of inhibition ≥ 12 mm against both Gram-positive and Gram-negative bacteria. The minimum inhibitory concentration was calculated only with methanolic extracts of selected plants, those showed zone of inhibition ≥ 12 mm against both Gram-positive and Gram-negative bacteria. Several extracts had minimum inhibitory concentration ≤ 1 mg/mL. Specifically Adhatoda vasica, Ageratum conyzoides, Alangium salvifolium, Alpinia galanga, Andrographis paniculata, Anogeissus latifolia, Annona squamosa, A. reticulate, Azadirachta indica, Buchanania lanzan, Cassia fistula, Celastrus paniculatus, Centella asiatica, Clausena excavate, Cleome viscosa, Cleistanthus collinus, Clerodendrum indicum, Croton roxburghii, Diospyros melanoxylon, Eleutherine bulbosa, Erycibe paniculata, Eryngium foetidum, Garcinia cowa, Helicteres isora, Hemidesmus indicus, Holarrhena antidysenterica, Lannea coromandelica, Millettia extensa, Mimusops elengi, Nyctanthes arbor-tristis, Oroxylum indicum, Paederia foetida, Pterospermum acerifolium, Punica granatum, Semecarpus anacardium, Spondias pinnata, Terminalia alata and Vitex negundo were shown to have significant antimicrobial activity. The species listed here were shown to have anti-infective activity against both Gram-positive and Gram-negative bacteria. These results may serve as a guide for selecting plant species that could yield the highest probability of finding promising compounds responsible for the antibacterial activities against a broad spectrum of bacterial species. Further investigation of the phytochemicals from these plants will help to identify the lead compounds for drug discovery.
Xanthones from the Leaves of Garcinia cowa Induce Cell Cycle Arrest, Apoptosis, and Autophagy in Cancer Cells.[Pubmed: 26102071]
Two new xanthones, cowaxanthones G (1) and H (2), and 23 known analogues were isolated from an acetone extract of the leaves of Garcinia cowa. The isolated compounds were evaluated for cytotoxicity against three cancer cell lines and immortalized HL7702 normal liver cells, whereby compounds 1, 5, 8, and 15-17 exhibited significant cytotoxicity. Cell cycle analysis using flow cytometry showed that 5 induced cell cycle arrest at the S phase in a dose-dependent manner, 1 and 16 at the G2/M phase, and 17 at the G1 phase, while 16 and 17 induced apoptosis. Moreover, autophagy analysis by GFP-LC3 puncta formation and western blotting suggested that 17 induced autophagy. Taken together, our results suggest that these xanthones possess anticancer activities targeting cell cycle, apoptosis, and autophagy signaling pathways.
Kaennacowanols A-C, three new xanthones and their cytotoxicity from the roots of Garcinia cowa.[Pubmed: 25771120]
Three new xanthones, named kaennacowanols A-C (1-3), along with nineteen known xanthones were isolated from the roots of Garcinia cowa Roxb. Their structures were determined by spectroscopic analysis. All isolated compounds were evaluated for their cytotoxicity against KB and HeLa cell lines. Compounds 17 and 22 showed good cytotoxicity against KB cell with IC50 values of 7.97 and 9.10μM, respectively. On the other hand, compound 15 showed good cytotoxicity against HeLa cell with IC50 value of 9.34μM.
Bioactive prenylated xanthones from the young fruits and flowers of Garcinia cowa.[Pubmed: 25651042]
Five new xanthones, garciniacowones A-E (1-5), together with 14 known xanthones, 6-19, were isolated from the young fruits and fresh flowers of Garcinia cowa. The structures of 1-5 were elucidated by analysis of their 1D and 2D NMR spectra and mass spectrometric data. The compounds 1-19 were tested in vitro for their antimicrobial activity and for their ability to inhibit α-glucosidase. Compounds 16 and 17 showed the most potent α-glucosidase inhibitory activity, with IC50 values of 7.8 ± 0.5 and 8.7 ± 0.3 μM, respectively. Compounds 8, 9, and 19 showed antibacterial activity against Bacillus subtilis TISTR 088 with identical MIC values of 2 μg/mL, while 8, 10, and 19 exhibited antibacterial activity against Bacillus cereus TISTR 688 with identical MIC values of 4 μg/mL.
Antioxidant and antiplatlet aggregation properties of bark extracts of Garcinia pedunculata and Garcinia cowa.[Pubmed: 25114359]
The bark extracts of Garcinia pedunculata and Garcinia cowa, which are abundant in the Northeastern regions of India, were screened for their antioxidant and in vitro antiplatelet aggregating activities. By β-carotene linoleate model for antioxidant assay, acetone extract of G. pedunculata and hexane extracts of G. cowa exhibited higher antioxidant activity (86.47 and 66.94 % respectively, at 25 ppm) than other extracts. Similar pattern was observed for superoxide radical scavenging method for antioxidant assay. The ethyl acetate extract of G. pedunculata and hexane extract of G. cowa exhibited higher antiplatelet aggregation capacity towards ADP induced platelet aggregation (IC50 0.16 and 0.43 ug, respectively) than other extracts.
Antibacterial tetraoxygenated xanthones from the immature fruits of Garcinia cowa.[Pubmed: 25110196]
A phytochemical investigation of the acetone extract from the immature fruits of Garcinia cowa led to the isolation of two novel tetraoxygenated xanthones, garcicowanones A (1) and B (2), together with eight known tetraoxygeanted xanthones. Their structures were determined by spectroscopic analysis. All isolated compounds were evaluated for their antibacterial activity against Bacillus cereus TISTR 688, Bacillus subtilis TISTR 008, Micrococcus luteus TISTR 884, Staphylococcus aureus TISTR 1466, Escherichia coli TISTR 780, Pseudomonas aeruginosa TISTR 781, Salmonella typhimurium TISTR 292 and Staphylococcus epidermidis ATCC 12228. α-Mangostin showed potent activity (MIC 0.25-1 μg/mL) against three Gram-positive strains and garcicowanone A and β-mangostin exhibited strong antibacterial activity against B. cereus with the same MIC values of 0.25 μg/mL.
Cowabenzophenones A and B, two new tetracyclo[7.3.3.3(3,11).0(3,7)]tetradecane-2,12,14-trione derivatives, from ripe fruits of Garcinia cowa.[Pubmed: 24334104]
Two new tetracyclo[7.3.3.3(3,11).0(3,7)]tetradecane-2,12,14-trione derivatives, cowabenzophenones A (1) and B (2), were isolated from ripe fruits of Garcinia cowa Roxb. Their structures were determined by spectroscopic methods. The tetracyclo[7.3.3.3(3,11).0(3,7)]tetradecane-2,12,14-trione skeleton from the Garcinia genus is reported for the first time.


