Gentianella azurea
Gentianella azurea
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Gentianella azurea
- Cat.No. Product Name CAS Number COA
-
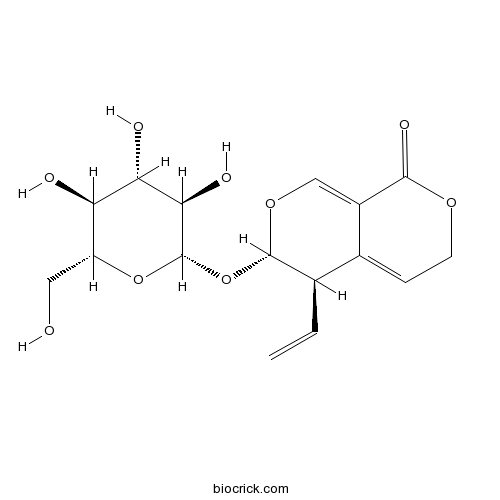
BCN4909
Gentiopicroside20831-76-9
Instructions
-
BCN2759
Swertianolin23445-00-3
Instructions

-
BCN4327
Ursolic acid77-52-1
Instructions

Anti-inflammatory secoiridoid glycosides from Gentianella azurea.[Pubmed: 25442320]
A phytochemical investigation on crude extract of Gentianella azurea led to the isolation of ten new (1-10) and one known (11) secoiridoid glycosides. Their structures were unambiguously elucidated by analysis of 1D and 2D NMR. Compounds 2, 5 and 11 were found to inhibit nitric oxide (NO) production in RAW 264.7 macrophages with IC50 values of 52.78 ± 8.61, 0.69 ± 0.23 and 5.18 ± 1.33, respectively, while indomethacin, the positive control, showed an IC50 value of 1.25 ± 0.52 μM.
Dammarane-type triterpenoids from Gentianella azurea.[Pubmed: 24806310]
Thirteen new dammarane-type triterpenoids (1-13) and four known analogues, gentirigenic acid (14) and the gentirigeosides A, B, and E (15-17), were isolated from Gentianella azurea. Their structures were elucidated by detailed analysis of the NMR, MS, and X-ray crystallographic data. This is the first report of dammarane-type triterpenoids in the Gentianella genus. In addition, the known structures of gentirigenic acid (14) and the gentirigeosides A, B, and E (15-17) were revised based on the X-ray diffraction analysis. Gentirigeoside A (15) was found to inhibit nitric oxide production in RAW 264.7 macrophages with an IC50 value of 6.6 ± 2.1 μM.


