Gymnema sylvestre
Gymnema sylvestre
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
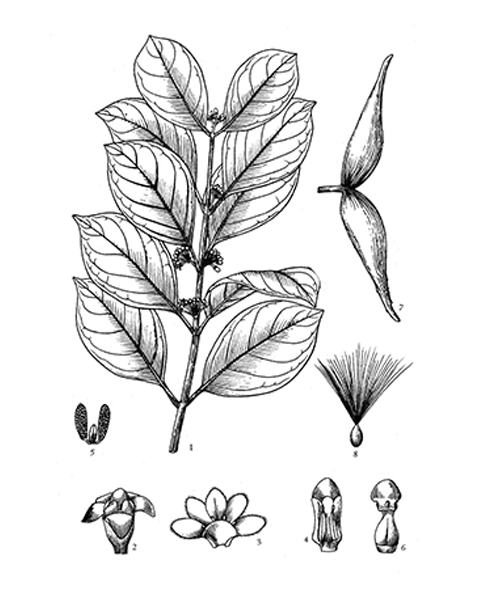
Natural products/compounds from Gymnema sylvestre
- Cat.No. Product Name CAS Number COA
-
BCN1040
Epimedin C110642-44-9
Instructions

-
BCN5938
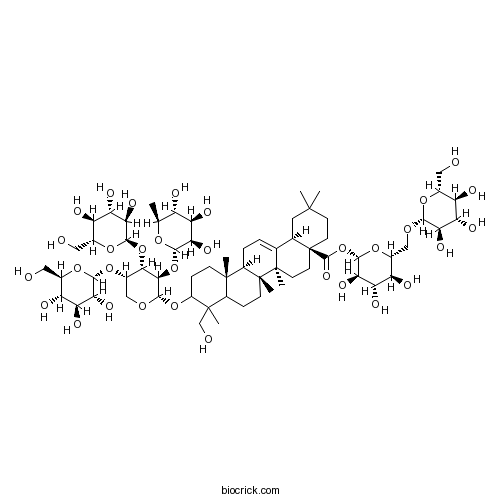
Macranthoidin B136849-88-2
Instructions
-
BCN2808
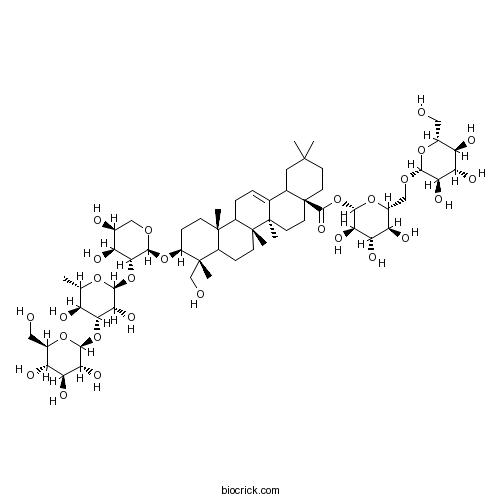
Macranthoidin A140360-29-8
Instructions
-
BCN1673
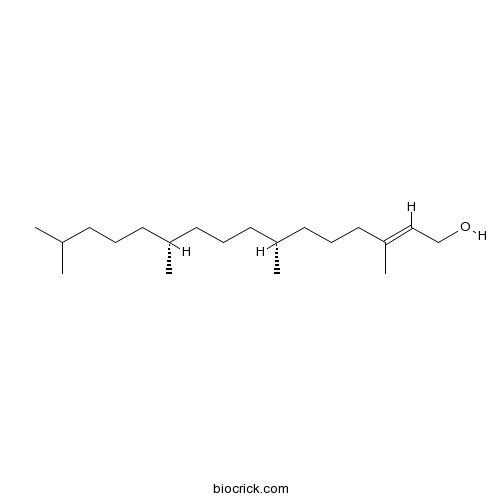
Phytol150-86-7
Instructions
-
BCN7854
21-O-Tigloylgymnemagenin1581276-63-2
Instructions

-
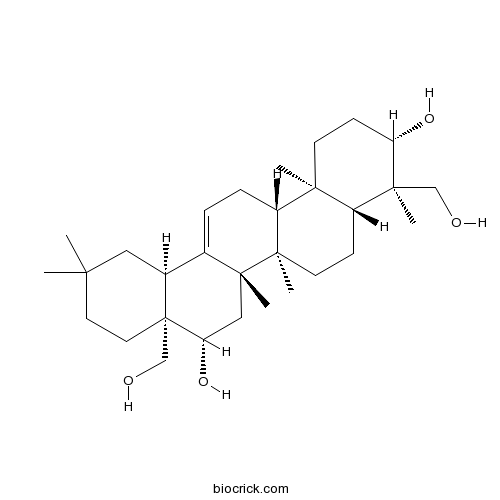
BCN7846
Gymnestrogenin19942-02-0
Instructions
-
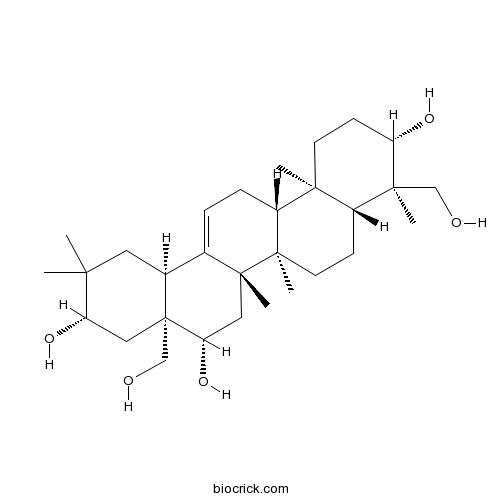
BCN7841
Gymnemagenin22467-07-8
Instructions
-
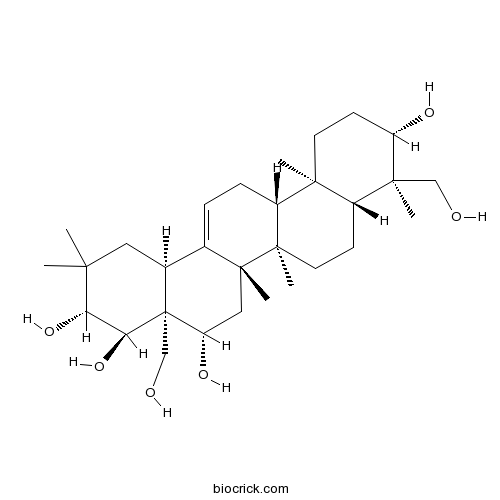
BCN7830
23-Hydroxylongispinogenin42483-24-9
Instructions
-
BCN6532
Neoisoastilbin54141-72-9
Instructions

Sweet taste receptor inhibitors: Potential treatment for equine insulin dysregulation.[Pubmed: 29958298]
Hyperinsulinemia is a major risk factor for equine laminitis, a debilitating and painful foot condition. Sweet taste receptor (T1R2/3) inhibitors have been used to reduce the insulin and glucose responses to oral carbohydrates in other species. However, their effect in horses has not been investigated. It would be useful to be able to attenuate the large post-prandial insulin response that typically occurs when a carbohydrate-rich meal is fed to insulin-dysregulated horses. Here we have determined the efficacy of two T1R2/3 inhibitors, lactisole and Gymnema sylvestre, for reducing glucose uptake by the equine small intestine in vitro; and post-prandial insulin secretion in ponies in vivo, following a carbohydrate-based meal. We used gas chromatography-mass spectrometry to measure 2-deoxyglucose uptake by explants of small intestine, in the presence and absence of the T1R2/3 inhibitors. Lactisole and G sylvestre reduced 2-deoxyglucose uptake by the intestinal explants by 63% (P = 0.032) and 73% (P = 0.047), respectively, compared to control samples. The study in vivo investigated the effect of the inhibitors on the blood glucose and serum insulin responses to a meal containing D-glucose. Three doses of each inhibitor were tested using a Latin square design, and each dose was compared to a meal with no inhibitor added. Lactisole had no effect on glucose and insulin concentrations, whereas G sylvestre was partially effective at reducing post-prandial blood glucose (by ~10%) and serum insulin concentrations (~25%) in seven ponies, with a most effective dose of 10 mg/kg bodyweight. These data provide preliminary support that T1R2/3 inhibitors may be a useful therapeutic strategy for the management of equine insulin dysregulation and the prevention of laminitis. However, further optimisation of the dose and delivery method for these compounds is required, as well as a direct investigation of their activity on the equine sweet taste receptor.
Flavor Alterations Associated with Miracle Fruit and Gymnema sylvestre.[Pubmed: 29905783]
Taste and flavor (retronasal olfaction) interact in the brain. The rules of that interaction are not well understood. This study uses 2 taste modifiers that alter sweet to examine the effects on flavors. Subjects used the Global Sensory Intensity Scale to assess the aroma, sweetness, sourness, and flavor of 10 foods. As previous work had shown, miracle fruit added sweetness to acids, which secondarily reduced sourness (mixture suppression) and Gymnema sylvestre reduced sweetness in sweet foods as well as the sweetness induced by miracle fruit. In this study, multiple regression showed that both sweet and sour contribute to flavor. Gymnema sylvestre reduced the perceived sweet of predominantly sweet foods (chocolate and maple syrup) as expected; reducing the sweet, reduced the flavor. The effects of miracle fruit were complicated by its dual action: intensification of sweet and reduction of sour. Predominantly sour foods (vinegar, lemon, mustard, pickle) were sweetened by miracle fruit but any flavor enhancement associated with the added sweet appears to have been countered by the flavor reduction associated with reduced sourness. Moderately sour foods that are also sweet (tomatoes, strawberries) were sweetened by miracle fruit and thus flavor was enhanced; flavor loss through sour reduction was apparently not sufficient to counter the flavor enhancement due to increased sweet so the net result was that tomato and strawberry flavors were enhanced. The flavors of control foods (not predominantly sweet or sour [sausage, peanuts]) showed only small changes.
Biosynthesis and production of quercitols and their application in the production of pharmaceuticals: current status and prospects.[Pubmed: 29663050]
(-)-vibo-Quercitol is a deoxyinositol (1L-1,2,4/3,5-cyclohexanepentol) that occurs naturally in low concentrations in oak species, honeydew honey, and Gymnema sylvestre. The author's research group recently reported that (-)-vibo-quercitol and scyllo-quercitol (2-deoxy-myo-inositol, 1,3,5/2,4-cyclohexanepentol), a stereoisomer of (-)-vibo-quercitol, are stereoselectively synthesized from 2-deoxy-scyllo-inosose by the reductive reaction of a novel (-)-vibo-quercitol 1-dehydrogenase in Burkholderia terrae and of a known scyllo-inositol dehydrogenase in Bacillus subtilis, respectively. The author's research group therefore identified two enzymes capable of producing both stereoisomers of deoxyinositols, which are rare in nature. (-)-vibo-Quercitol and scyllo-quercitol are potential intermediates for pharmaceuticals. In this review, the author describes the biosynthesis and enzymatic production of quercitols and myo-inositol stereoisomers and their application in the production of potential pharmaceuticals.
Discrimination of different geographic varieties of Gymnema sylvestre, an anti-sweet plant used for the treatment of type 2 diabetes.[Pubmed: 29529525]
Gymnema sylvestre (Retz.) R.Br. ex Sm. (Asclepiadaceae) is a well-known Ayurvedic anti-sweet plant for the treatment of type 2 diabetes mellitus. Although it was previously proposed that G. sylvestre exhibits chemical variation based on geography, most research on G. sylvestre has used material originating from India. Morphological and anatomical descriptions, ITS1-5.8S-ITS2 DNA sequencing, and acid hydrolysis analyses showed that G. sylvestre samples from Vietnam are distinguishable from those of Indian origin and thus suggest a dissimilarity among G. sylvestre samples with different geographic distributions. An LC-MS-guided strategy targeting 3β-glucuronide oleane-triterpenes in the Vietnamese G. sylvestre variety led to the isolation of four known compounds and nine previously undescribed compounds, named gymnemosides ND1-ND9. None of the isolated compounds were reported in the Indian sample, further supporting the geo-diversity of G. sylvestre. Three compounds, gymnemosides ND7-9, exerted significant stimulatory effects on the uptake of 2-NBDG in 3T3-L1 adipocyte cells and thus have potential as lead molecules for anti-diabetes agents.
In vivo pharmacokinetic interaction by ethanolic extract of Gymnema sylvestre with CYP2C9 (Tolbutamide), CYP3A4 (Amlodipine) and CYP1A2 (Phenacetin) in rats.[Pubmed: 29042257]
None
In vitro Inhibitory Effect of Gymnema sylvestre Extracts and Total Gymnemic Acids Fraction on Select Cytochrome P450 Activities in Rat Liver Microsomes.[Pubmed: 29019074]
Gymnema sylvestre R. Br. is a well-known Indian medicinal herb. Gymnemic acids are pentacyclic triterpenes saponins and active phytoconstituents of Gymnema sylvestre. The study aimed at evaluation of the in vitro rat liver cytochrome P450 (CYP) inhibition potential of extracts and total gymnemic acid (TA)-enriched fractions from G. sylvestre.
Identification and characterization of a novel (-)-vibo-quercitol 1-dehydrogenase from Burkholderia terrae suitable for production of (-)-vibo-quercitol from 2-deoxy-scyllo-inosose.[Pubmed: 28905086]
(-)-vibo-Quercitol is a deoxyinositol (1L-1,2,4/3,5-cyclohexanepentol) that naturally occurs in oak species, honeydew honey, wines aged in oak barrels, and Gymnema sylvestre and is a potential intermediate for pharmaceuticals. We found that (-)-vibo-quercitol is stereoselectively synthesized from 2-deoxy-scyllo-inosose by the reductive reaction of a novel (-)-vibo-quercitol 1-dehydrogenase found in the proteomes of Burkholderia, Pseudomonas, and Arthrobacter. Among them, Burkholderia terrae sp. AKC-020 (40-1) produced a (-)-vibo-quercitol 1-dehydrogenase appropriate for synthesizing (-)-vibo-quercitol with a high diastereomeric excess. The enzyme was strongly induced in Bu. terrae cells when quercitol or 2-deoxy-scyllo-inosose was used as carbon source in the culture medium. The enzyme is NAD(H)-dependent and shows highly specific activity for (-)-vibo-quercitol and myo-inositol among the substrates tested. The enzyme gene (qudh) was obtained by PCR using degenerate primers based on the N-terminal and internal amino acid sequences of the purified enzyme, followed by thermal asymmetric interlaced PCR. The qudh gene showed homology with inositol 2-dehydrogenase (sharing 49.5% amino acid identity with IdhA from Sinorhizobium meliloti 1021). We successfully produced several recombinant (-)-vibo-quercitol 1-dehydrogenases and related enzymes identified by genome database mining using an Escherichia coli expression system. This revealed that scyllo-inositol dehydrogenase (IolX) in Bacillus subtilis can catalyze the reduction of 2-deoxy-scyllo-inosose to yield scyllo-quercitol, a stereoisomer of (-)-vibo-quercitol. Thus, we successfully identified two enzymes to produce both stereoisomers of deoxyinositols that are rare in nature.


