Hovenia trichocarpa
Hovenia trichocarpa
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Hovenia trichocarpa
- Cat.No. Product Name CAS Number COA
-
BCN5927
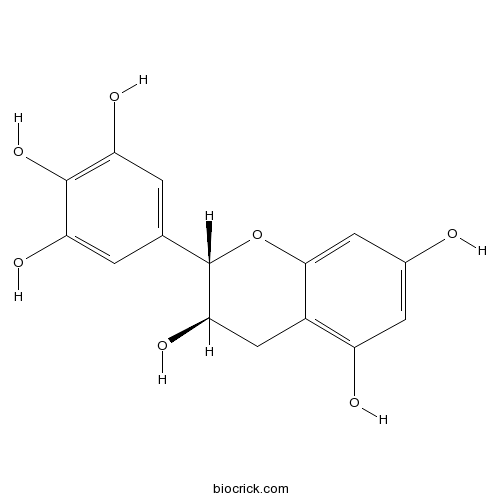
(-)-Gallocatechin3371-27-5
Instructions
Two novel saponins of 20, 26-epoxy derivatives of pseudojujubogenin from the seeds of Hovenia trichocarpa.[Pubmed: 23529014]
Two new saponins of 20, 26-epoxy derivatives of pseudojujubogenin, hoduloside XI (1) and hoduloside XII (2) were isolated from the seeds of Hovenia trichocarpa. The structures of the new compounds were established by extensive NMR experiments and chemical methods. Hoduloside XI was confirmed to be 3-O-{β-D-glucopyranosyl(1→3)-[β-D-xylopyranosyl(1→2)]-α-L-arabinopyranosyl}-20, 26-epoxypseudojujubogenin. Hoduloside XII was identified as 3-O-{β-D-xylopyranose(1→2)glucopyranosyl(1→3)[rhamnopyranose(1→2)]β-D-glucopyranosyl}-20, 26-epoxypseudojujubogenin. The in vitro cytotoxic activity of compounds 1 and 2 was assayed. They displayed inhibitive activities against human cancer cell lines HL60 and K562.
Isolation and absolute structures of enantiomeric 1,2-bis(4-hydroxy-3-methoxyphenyl)-1,3-propanediol 1-O-glucosides from the bark of Hovenia trichocarpa.[Pubmed: 9748383]
Two 1,2-bis(4-hydroxy-3-methoxyphenyl)-1,3-propanediol 1-O-glucosides, hovetrichosides A (1) and B (2), were isolated from the bark of Hovenia trichocarpa. Their structures were established by extensive NMR experiments and chemical methods. Compounds 1 and 2 were (1R), (2S)-1-(4-hydroxy-3-methoxyphenyl)-2-(4-hydroxy-3-methoxyphenyl)-1, 3-propanediol 1-O-beta-D-glucopyranoside and (1S), (2R)-1-(4-hydroxy-3-methoxyphenyl)-2-(4-hydroxy-3-methoxyphenyl)-1, 3-propanediol 1-O-beta-D-glucopyranoside, respectively.


