Incarvillea mairei
Incarvillea mairei
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Incarvillea mairei
- Cat.No. Product Name CAS Number COA
-
BCN2864
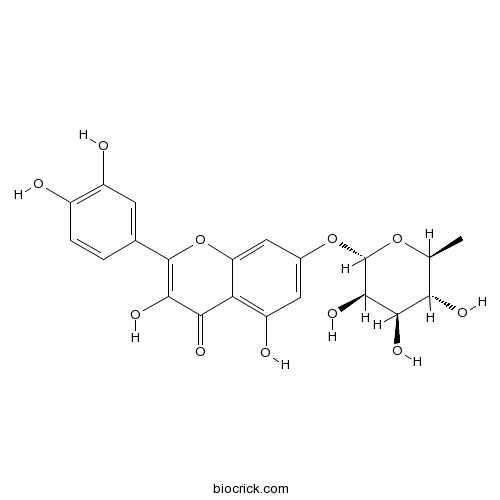
Vincetoxicoside B22007-72-3
Instructions
Total synthesis of incargranine A.[Pubmed: 29638233]
Synthetic studies into the origins of the alkaloid incargranine A have resulted in the development of a four-step (longest linear sequence) total synthesis. This synthesis has been scaled-up to provide gram-scale quantities of material, which would alternatively require extraction of several metric-tons of dried-whole Chinese Trumpet-Creeper plants (Incarvillea mairei var. grandiflora).
Neuroprotection of (+)-2-(1-Hydroxyl-4-Oxocyclohexyl) Ethyl Caffeate Against Hydrogen Peroxide and Lipopolysaccharide Induced Injury via Modulating Arachidonic Acid Network and p38-MAPK Signaling.[Pubmed: 26510982]
Oxidative stress and neuroinflammation are highly relevant to the pathological processes of various neurodegenerative diseases including Alzheimer's disease (AD). (+)-2-(1-hydroxyl-4-oxocyclohexyl) ethyl caffeate (HOEC), a novel 5-lipoxygenase inhibitor, was isolated from the whole plant of Incarvillea mairei var granditlora (Wehrhahn) Grierson. In this study, we investigated the protective effect of HOEC on hydrogen peroxide (H2O2) and lipopolysaccharide (LPS) -induced cytotoxicity and neuroinflammation in vitro and in vivo. MTT assay, LDH release assay, morphological observation and Hoechst 33342/PI dual staining followed by EIA, immunofluorescence staining and Western Blotting analysis were performed to elucidate the neuroprotective effect of HOEC. Treatment with HOEC at various concentrations prior to H2O2 exposure significantly enhanced cell viability, decreased LDH release, prevented cell morphologic changes and apoptosis. Instead of PGE2 reduction, HOEC markedly inhibited the production of LTB4 and suppressed the macrophage-mediated neurotoxicity. Western blotting and immunofluorescence staining showed that HOEC inhibited H2O2-induced p38 phosphorylation and NF-κB activation. Neuroprotective effect of HOEC was abolished by a p38 inhibitor. Further in vivo studies of LPS-induced neuroinflammation confirmed the anti-inflammatory effects of HOEC. These findings that HOEC protects SH-SY5Y cells from H2O2 and LPS-induced injury via arachidonic acid network modulation followed by p38 MAPK and NF-κB signaling, might make HOEC be considered as a therapeutic candidate for prevention and treatment of neurodegenerative diseases involving oxidative stress or/and inflammation.
Protective effects of (E)-2-(1-hydroxyl-4-oxocyclohexyl) ethyl caffeine against hydrogen peroxide-induced injury in PC12 cells.[Pubmed: 25503480]
(E)-2-(1-hydroxyl-4-oxocyclohexyl) ethyl caffeine (HOEC), a naturally caffeic ester isolated from Incarvillea mairei, has been reported to possess anti-inflammatory activity by targeting 5-lipoxygenase. However, its other potential activities have yet to be explored. In this study, we measured antioxidant activity of HOEC using the DPPH free radical-scavenging assay. Then, we exposed rat pheochromocytoma (PC12) cells to hydrogen peroxide (H2O2)-induced damage and investigated the antioxidant activity of HOEC. Cell viability, lactate dehydrogenase (LDH) release, cellular morphology, Hoechst 33342 fluorescent staining, and apoptosis of the PC12 cells were assessed after treatment with 0.3-10 μM HOEC for 2 h and exposure to 600 μM H2O2. Additionally, glutathione reductase (GR), superoxide dismutase (SOD), lipid peroxidation malondialdehyde (MDA), and intracellular reactive oxygen species (ROS) accumulation were assayed after the PC12 cells were exposed to H2O2. To investigate mechanism, apoptosis-related protein were evaluated, including cleaved caspase 3/7, cleaved PARP, Bcl-2, Bcl-XL, and cytochrome c. The results showed that HOEC possessed potent antioxidant activity and pre-treatment with HOEC prior to H2O2 exposure significantly increased cell viability, reduced the release of LDH, ameliorated changes in cell morphology, and inhibited apoptosis. Further, HOEC did the following: reduced intracellular accumulation of ROS and MDA; rescued loss of SOD and GR activities; inhibited activated caspase-3 and caspase-7, cleaved PARP, and cytochrome c release; up-regulated the antiapoptosis-related protein Bcl-2 and Bcl-XL; and down-regulated the apoptosis-related proteins Bax and Bad. These findings suggested that HOEC may be a therapeutic agent for treating oxidative stress-derived neurodegenerative disorders.
(+)-2-(1-Hydroxyl-4-oxocyclohexyl) ethyl caffeate suppresses solar UV-induced skin carcinogenesis by targeting PI3K, ERK1/2, and p38.[Pubmed: 24845061]
For decades, skin cancer incidence has increased, mainly because of oncogenic signaling pathways activated by solar ultraviolet (UV) irradiation (i.e., sun exposure). Solar UV induces multiple signaling pathways that are critical in the development of skin cancer, and therefore the development of compounds capable of targeting multiple molecules for chemoprevention of skin carcinogenesis is urgently needed. Herein, we examined the chemopreventive effects and the molecular mechanism of (+)-2-(1-hydroxyl-4-oxocyclohexyl) ethyl caffeate (HOEC), isolated from Incarvillea mairei var. grandiflora (Wehrhahn) Grierson. HOEC strongly inhibited neoplastic transformation of JB6 Cl41 cells without toxicity. PI3K, ERK1/2, and p38 kinase activities were suppressed by direct binding with HOEC in vitro. Our in silico docking data showed that HOEC binds at the ATP-binding site of each kinase. The inhibition of solar UV-induced PI3K, ERK1/2, and p38 kinase activities resulted in suppression of their downstream signaling pathways and AP1 and NF-κB transactivation in JB6 cells. Furthermore, topical application of HOEC reduced skin cancer incidence and tumor volume in SKH-1 hairless mice chronically exposed to solar UV. In summary, our results show that HOEC exerts inhibitory effects on multiple kinase targets and their downstream pathways activated by solar UV in vitro and in vivo. These findings suggest that HOEC is a potent chemopreventive compound against skin carcinogenesis caused by solar UV exposure.
The reproductive strategy of a pollinator-limited Himalayan plant, Incarvillea mairei (Bignoniaceae).[Pubmed: 24289097]
Plants may adapt to alpine habitats by specialization in the reproductive strategy and functional aspects of their flowers and pollination systems. Alpine habitats reduce the opportunities for cross-pollination in a relatively high proportion of alpine plant species, and self-pollination may be favored in these adverse conditions. Here, we investigated the mating system and pollination of Incarvillea mairei, a perennial Himalayan herb typically found at altitudes between 3000 and 4500 m.
Cyclohexyl-ethanol derivatives from the roots of Incarvillea mairei.[Pubmed: 20183285]
Two new cyclohexyl-ethanol derivatives, incarvmareins A (1) and B (2), together with two known derivatives, 3 and 4, were isolated from the ethanolic extract of the roots of Incarvillea mairei. The structures of the new compounds were elucidated primarily on the basis of analysis of spectroscopic data.
[Chemical constituents from roots of Incarvillea mairei].[Pubmed: 19873778]
To study the chemical constituents of the roots of Incarvillea mairei.


