Isodon inflexus
Isodon inflexus
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Isodon inflexus
- Cat.No. Product Name CAS Number COA
-
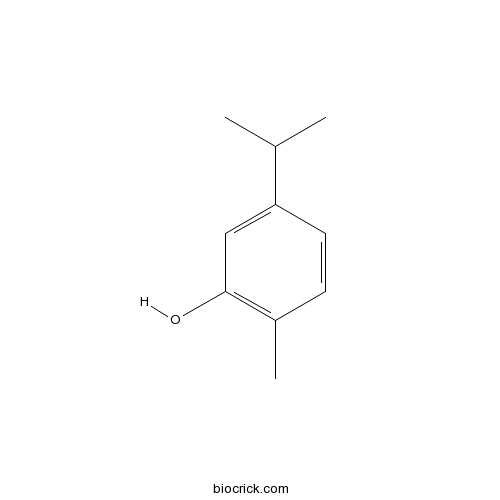
BCN2633
5-Isopropyl-2-methylphenol499-75-2
Instructions
-
BCN4327
Ursolic acid77-52-1
Instructions

A new kaurane diterpenoid from Isodon inflexus.[Pubmed: 26461051]
A new 7,20-epoxy kaurane diterpenoid, 15-acetyldemethylkamebacetal A (1) and six known kaurane diterpenoids (2-7) were isolated from the aerial parts of Isodon inflexus in nuclear transcription factor-κB (NF-κB)-dependent reporter gene assay-guided fractionation. Their chemical structures were determined on the basis of extensive spectroscopic analysis (UV, IR, MS, 1D- and 2D-NMR) and comparison with literature data. The isolated compounds were evaluated for their inhibitory effects on TNF-α-induced NF-κB activation, and all compounds exhibited NF-κB inhibitory activities with IC50 values ranging from 1.91 to 20.15 μM.
Abietane diterpenoids from Isodon inflexus.[Pubmed: 22766206]
Four abietane diterpenoids, inflexanin C, inflexanin D, inflexuside A and inflexuside B, were isolated from the aerial parts of Isodon inflexus. Their respective structures were established by NMR, mass spectrometry and CD as (+)-(1S,4R,5S,7S,8S,10S,13S)-1,7,18-trihydroxy-abieta-9(11)-ene-12-one 1-monoacetate, (+)-(1S,4R,5S,10S,13S)-1,18-dihydroxy-abieta-7,9(11)-diene-12-one 1-monoacetate, (-)-(1S,5S,10S,11R,13R)-1,11,13-trihydroxy-abieta-8-ene-7-one 1-O-β-D-glucopyranoside and (-)-(1S,5S,10S,11R,13R)-1,11,13-trihydroxy-abieta-8-ene-7-one 1-O-(2-O-coumaroyl)-β-D-glucopyranoside. All compounds showed strong inhibitory activity against nitric oxide (NO) production in RAW264.7 lipopolysaccaride (LPS)-activated macrophages.
A new abietane diterpenoid from Isodon inflexus.[Pubmed: 19023532]
A new ent-abietane diterpenoid, 3alpha,6beta-dihydroxy-7,17-dioxo-ent-abieta-15(16)-ene (1), and three known ent-kaurane diterpenids, kamebacetal A (2), kamebakaurin (3), and excisanin A (4), and a known triterpenoid, ursolic acid (5), were isolated from the aerial parts of Isodon inflexus. Their chemical structures were determined by extensive analysis of spectroscopic data including 1D-and 2D-NMR experiments. All isolates (1-5) were evaluated for their potential to inhibit LPS-induced nitric oxide production in RAW264.7 cells. Of these, compounds 1-4 inhibited the production of NO with IC(50) values ranging from 1.0 to 26.5 microM.
[IR-fingerprinting ordination comparison on different organs of three species of isodon].[Pubmed: 12914173]
The plants of the genus Isodon are of important medicinal values, being widely used in the production of the Chinese traditional and herbal drugs. Compared with the other identification methods, the identification of the Chinese traditional and herbal drugs using the fourier-transform infrared spectrometer with OMNI collector is simple and convenient, fast and accurate. Moreover, the extraction or break of the samples are not necessary in the identification using OMNI collector. In the present paper, fourier-transform infrared spectrometer with OMNI collector is applied to gain the IR-fingerprintings of eleven samples of Isodon inflexus, I. Lophanthoides and I. Macrocalyx. Based on the indices of wavenumber-absorbance, the differences of eleven IR-fingerprintings are compared by PCA (Principal Component Analysis). The results show that it is practical to apply PCA on the basis of IR-fingerprinting to compare the chemical differences of plant samples.


