Lindera glauca
Lindera glauca
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Lindera glauca
- Cat.No. Product Name CAS Number COA
-
BCN6285
Norisoboldine23599-69-1
Instructions

-
BCN6315
Procyanidin B229106-49-8
Instructions

-
BCN5597
Epicatechin490-46-0
Instructions
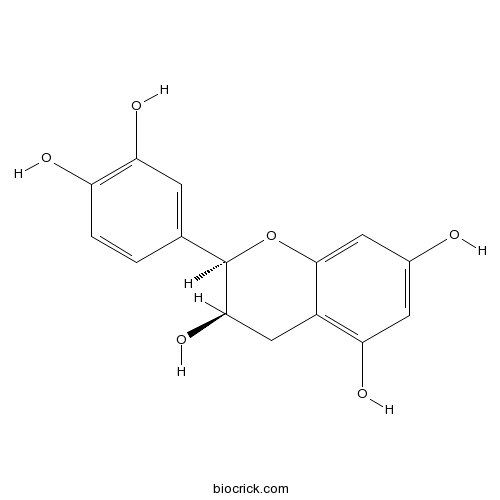
A new cerebroside from the twigs of Lindera glauca (Sieb. et Zucc.) Blume.[Pubmed: 28778013]
None
De novo assembly and characterization of the floral transcriptome of an economically important tree species, Lindera glauca (Lauraceae), including the development of EST-SSR markers for population genetics.[Pubmed: 27553669]
None
A new rearranged eudesmane sesquiterpene and bioactive sesquiterpenes from the twigs of Lindera glauca (Sieb. et Zucc.) Blume.[Pubmed: 27620498]
None
Integrated transcriptome sequencing and dynamic analysis reveal carbon source partitioning between terpenoid and oil accumulation in developing Lindera glauca fruits.[Pubmed: 26446413]
Lindera glauca fruits (LGF) with the abundance of terpenoid and oil has emerged as a novel specific material for industrial and medicinal application in China, but the complex regulatory mechanisms of carbon source partitioning into terpenoid biosynthetic pathway (TBP) and oil biosynthetic pathway (OBP) in developing LGF is still unknown. Here we perform the analysis of contents and compositions of terpenoid and oil from 7 stages of developing LGF to characterize a dramatic difference in temporal accumulative patterns. The resulting 3 crucial samples at 50, 125 and 150 days after flowering (DAF) were selected for comparative deep transcriptome analysis. By Illumina sequencing, the obtained approximately 81 million reads are assembled into 69,160 unigenes, among which 174, 71, 81 and 155 unigenes are implicated in glycolysis, pentose phosphate pathway (PPP), TBP and OBP, respectively. Integrated differential expression profiling and qRT-PCR, we specifically characterize the key enzymes and transcription factors (TFs) involved in regulating carbon allocation ratios for terpenoid or oil accumulation in developing LGF. These results contribute to our understanding of the regulatory mechanisms of carbon source partitioning between terpenoid and oil in developing LGF, and to the improvement of resource utilization and molecular breeding for L. glauca.
Transcriptome analysis of distinct Lindera glauca tissues revealed the differences in the unigenes related to terpenoid biosynthesis.[Pubmed: 25576222]
The Lindera glauca, an economically and ecologically important tree species, has emerged as a novel potential plant for the intensive studies of essential oil owing to its characteristic aroma and medicinal property in distinct tissues. However, the transcriptome information and molecular research on this species is still unknown to date. To reveal the formation and accumulation mechanism of essential oil in distinct L. glauca tissues, it is crucial to analyze transcriptome and to identify the full repertoire of potential unigenes involved in terpenoid biosynthesis. In this paper, the transcriptomes of the roots, sarcocarps, stems, leaves and kernels of L. glauca were analyzed for the first time by using short-read sequencing technology (Illumina). A total of 27.2GB valid reads (the average length=92.7bp) was obtained from distinct L. glauca tissues, and then assembled de novo into 264,831 unigenes by Trinity strategy (mean size=560.2bp). The resulting 98,141 unigenes (38%) of all the assembled unigenes were annotated in multiple public databases, of which 114 potential unigenes were identified to be involved in the terpenoid biosynthetic accumulation in L. glauca. Additionally, the differential expression profiles revealed 675, 697, 432, 1702 and 844 high tissue-specificity expressions of unigenes in the roots, sarcocarps, stems, leaves and kernels of L. glauca, respectively. Overall, these obtained comprehensive unigene resources will contribute to advance the research regarding the specific plant and more specifically discovery of genes participating in the terpenoid pathway and its regulation in specific tissues of the L. glauca, but also could help the understanding of the differential accumulation of secondary metabolites in distinct plant tissues.
Bioactive lignan constituents from the twigs of Lindera glauca.[Pubmed: 25366316]
A bioassay-guided fractionation and chemical investigation of the MeOH extract from the twigs of Lindera glauca (SIEB. et ZUCC.) BLUME resulted in the isolation and identification of six lignans (1-6) including three new lignan derivatives, named linderuca A (1), B (2), and C (3). The structures of the new compounds (1-3) were determined on the basis of spectroscopic analyses, including two dimensional NMR and circular dichroism (CD) spectroscopy studies. The cytotoxic activities of the isolates (1-6) were evaluated by determining their inhibitory effects on human tumor cell lines. Compounds 1-5 showed antiproliferative activities against A549, SK-OV-3, SK-MEL-2, and HCT-15 cell lines with IC50 values of 7.79-29.42 µM. Based on the understanding that inflammation is a crucial cause of tumor progression, we also investigated the anti-inflammatory activities of the isolates (1-6) in the lipopolysaccharide-stimulated murine microglia BV-2 cell line by measuring nitric oxide (NO) levels. The new lignans (1-3) significantly inhibited NO production with IC50 values of 12.10, 9.48, and 9.87 µM, respectively, without cytotoxicity.
Flavonoids from Lindera glauca Blume as low-density lipoprotein oxidation inhibitors.[Pubmed: 24499267]
In order to identify antioxidant flavonoids from Lindera glauca Blume, we performed phytochemical analysis of L. glauca Blume heartwood and isolated eight flavonoids - lindeglaucol (1), lindeglaucone (2), cilicicone B (3), tamarixetin 3-O-α-L-rhamnoside (4), procyanidin A2 (5), cinnamtannin B1 (6), cinnamtannin D1 (7), and procyanidin A1 (8) - through repeated column chromatography over silica gel (SiO₂), octadecyl silica gel (ODS) and Sephadex LH-20. The chemical structures of compounds 1-8 were elucidated from spectroscopic data (NMR, IR and MS). The low-density lipoprotein oxidation inhibitory activities of the isolated compounds were evaluated in vitro by using the thiobarbituric acid reactive substances assay. Compounds 5-8 exhibited high inhibition activity, comparable to the positive control butyl hydroxyl toluene. Compounds 2 and 3 were slightly less active, while 1 and 4 expressed low activity.


