Litsea cubeba
Litsea cubeba
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Litsea cubeba
- Cat.No. Product Name CAS Number COA
-
BCN9068
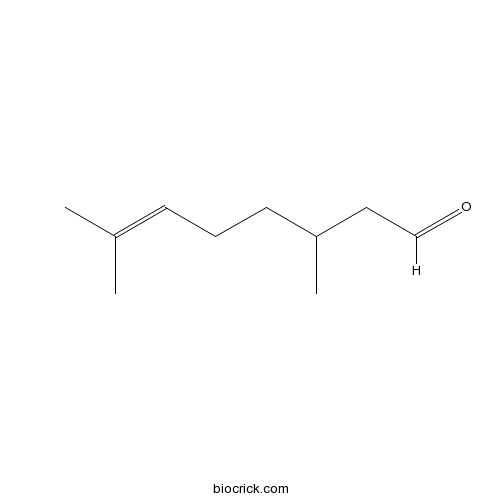
Citronellal106-23-0
Instructions
-
BCN2361
(+)-Isocorynoline475-67-2
Instructions

-
BCN9066
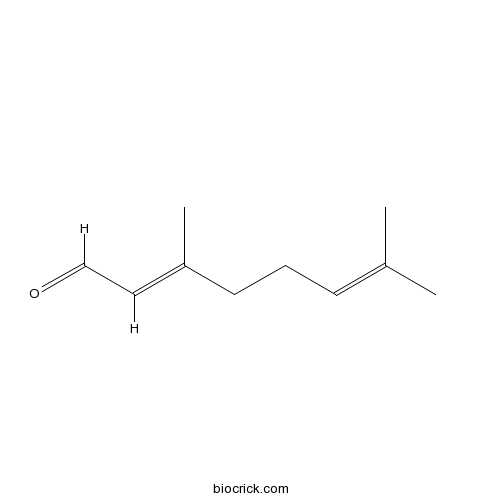
Citral5392-40-5
Instructions
-
BCN4213
N-trans-Feruloyltyramine66648-43-9
Instructions

-
BCN2644
trans-Caryophyllene87-44-5
Instructions

Physical, antibacterial and antioxidant properties of chitosan films containing hardleaf oatchestnut starch and Litsea cubeba oil.[Pubmed: 29959016]
None
Biosynthesis of SnO₂ Nanoparticles Based on Response Surface Methodology and the Study of Their Dye Removal.[Pubmed: 29442688]
Litsea cubeba is an evergreen tree from the Lauraceae family, with the common name of mountain pepper in Taiwan. The extracts from different parts such as the bark, leaf, root and fruit have various medicinal properties and have been extensively used in traditional Chinese medicine. SnO2 nanoparticles (NPs) were synthesized using an extract of Litsea cubeba fruit based on response surface methodology (RSM). The phytochemicals present in the extract of Litsea cubeba fruit played a crucial role in the NP formation, which could be confirmed by Fourier-Transform Infrared Spectrometer (FTIR). Analysis of variance (ANOVA) was used to evaluate the validation of the RSM models and indicated that the quadratic model was highly significant and suitable to represent the response of the NP formation yield. The optimum parameters were determined using the Design Expert Program and were as follows: extract volume ratio 30.0%, pH 6.3 and reaction temperature 44.4 °C. Using the optimal reaction conditions from the central composite design (CCD) of RSM, the SnO2 NPs were prepared and characterized by field-emission scanning electron microscopy (FE-SEM), energy dispersive X-ray spectroscopy (EDX), transmission electron microscopy (TEM) and X-ray diffraction (XRD). The FE-SEM results showed that the SnO2 NPs were spherical, and XRD results showed that the NPs had a tetragonal crystal structure. Furthermore, the photodegradation kinetics of malachite green by the SnO2 NPs was represented well with a pseudo first-order model.
Litsea cubeba leaf essential oil from Vietnam: chemical diversity and its impacts on antibacterial activity.[Pubmed: 29266378]
The threat of bacterial resistance to antibiotics has created an urgent need to develop new antimicrobials. The aim of this study was to characterize the chemical diversity of Litsea cubeba leaf essential oil (EO) and its impacts on the antibacterial activity against pathogenic bacteria. Essential oils collected from seven provinces in North Vietnam (n = 25) were characterized by their high content in either 1,8-cineole or linalool. Linalool-type EOs were more effective against the eight bacterial strains tested than 1,8-cineole-type. Oil samples, LC19 (50% 1,8-cineole) and BV27 (94% linalool), were selected to investigate their antibacterial mechanisms against Escherichia coli. A strong bactericidal effect was observed after 4 and 2 h of exposure respectively. Microscopic analysis of treated E. coli cultures clearly showed that EOs caused changes in cell morphology, loss of integrity and permeability of the cell membrane, as well as DNA loss. However, the effects of both EOs were distinct. LC19 mostly affected cell membrane, led to a significant cell filamentation rate and altered cell width, whereas BV27 damaged cell membrane integrity leading to cell permeabilization and altered nucleoid morphology with the appearance of spot and visibly altered compaction.
Insecticidal activities of constituents of Litsea cubeba fruit extracts effective against the maize weevil (Coleoptera: Curculionidae).[Pubmed: 29117378]
In this study, we investigated the insecticidal activities, including contact toxicity, fumigant toxicity, and repellent activity, of Litsea cubeba fruit extracts against Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). The extracts, obtained by liquid-liquid extraction in n-hexane, ethyl acetate, chloroform, and water were analyzed by gas chromatography-mass spectrometry. Among the different extract types, chloroform extracts exhibited the strongest repellent, contact, and fumigant activities against S. zeamais. The main components of the chloroform extracts were identified as laurine (21.15%) and 2,6-diisopropyl aniline (16.14%), followed by chlorobutanol (10.54%), 3-O-methyl-N-acetyl-d-glucosamine (10.03%), and 6-methyl-5-hepten-2-one (8.33%). Among the identified components of the chloroform extracts, chlorobutanol showed the strongest fumigant toxicity (LD50 = 21.91 mg/liter), contact toxicity (LD50 = 54.25 µg/adult), and repellent activity against S. zeamais. These results indicate that L. cubeba fruit extracts possess natural insecticide-like activities against S. zeamais.
[Water soluble constituents from the twigs of Litsea cubeba].[Pubmed: 29098825]
Twenty five known aromatic glycosides (1-25) and three known sesquiterpene glycosides (26-28) have been isolated from the twigs of Litsea cubeba by using various chromatographic techniques. Their structures were identified by spectroscopic data analysis (MS, IR, 1D and 2D NMR) as (7S,8R)-dehydrodiconiferyl alcohol 4,9'-di-O-β-D-glucopyranoside(1),(7S,8R)-5-methoxydihydrodehydrodiconiferyl alcohol 4-O-β-D-glucopyranoside(2), (7S,8R)-urolignoside(3), (7R,8S)-dihydrodehydrodiconiferyl alcohol 4-O-β-D-glucopyranoside(4), saposide B(5), lanicepside A(6), matairesinol-4-O-β-D-glucopyranoside (7), tyraxjaponoside B(8), (+)-lyoniresinol-9'-O-β-D-glucopyranoside (9), alaschanisoside A (10), syringin (11), psoralenoside (12), isopsoralenoside (13), scopolin(14), 2,6-dimethoxy-4-hydroxyphenol-1-O-β-D-glucopyranoside (15), 3-hydroxy-4,5-dimethoxyphenyl-β-D-glucopyranoside (16), 2-(3,4-dihydroxyphenyl)ethyl-β-D-glucopyrnoside (17), 2-(4-dihydroxyphenyl)ethyl-β-D-glucopyranoside (18), (+)-catechin-7-O-β-D-glucopyranoside (19), 3'-O-methylepicatechin-7-O-β-D-glucopyranoside (20), kaempferitrin (21), quercetin-3-O-α-L-rhamnopyranside (22), kaempferol-3-O-β-D-glucopyranoside (23), kaempferol 3-O-β-D-glucopyranosyl(1→2)-O-β-D-galactopyr anoside-7-O-α-L-rhamnopyranoside (24), quercetin 3-O-α-L-rhamnopyranosyl(1→6)-O-β-D-glucopyranosyl(1→3)-O-α-L-rhamnopyranosyl(1→2)-O-β-D-glucopyranoside (25), staphylionoside D(26), vomifoliol 9-O-β-D-glucopyranoside (27), dihydrovomifoliol-O-β-D-glucopyranoside (28). Compounds 1-21 and 24-28 were obtained from this genus for the first time.
Boldine isolated from Litsea cubeba inhibits bone resorption by suppressing the osteoclast differentiation in collagen-induced arthritis.[Pubmed: 28826044]
To investigate the effect of boldine isolated from Litsea cubeba on collagen-induced arthritis (CIA) rats and explore the molecular mechanism predicted by network pharmacology.


