Nandina domestica
Nandina domestica
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Nandina domestica
- Cat.No. Product Name CAS Number COA
-
BCN5979
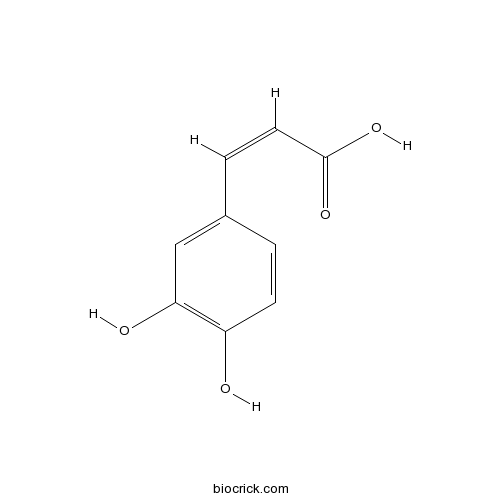
Caffeic acid331-39-5
Instructions
-
BCN4546
4-Hydroxybenzoic acid99-96-7
Instructions

Characterization of a novel hydroxynitrile lyase from Nandina domestica Thunb.[Pubmed: 29975178]
The leaves of Nandina domestica Thunb. exhibited high hydroxynitrile lyase (HNL) activity in (R)-mandelonitrile synthesis. The specific activity of young leaves was significantly higher than that of mature leaves. We isolated two HNLs with molecular mass of 24.9 kDa (NdHNL-S) and 28.0 kDa (NdHNL-L) from the young leaves. Both NdHNLs were composed of two identical subunits, without FAD and carbohydrates. We purified NdHNL-L and revealed its enzymatic properties. The whole deduced amino acid sequence of NdHNL-L was not homologous to any other HNLs, and the specific activity for mandelonitrile synthesis by NdHNL-L was higher than that by other plant HNLs. The enzyme catalyzed enantioselective synthesis of (R)-cyanohydrins, exhibited high activity at pH 4.0, and high stability in the pH range of 3.5-8.0 and below 55°C. Thus, NdHNL-L is a novel HNL with novel amino acid sequence and has a potential for the efficient production of (R)-cyanohydrins.
Determination of higenamine and coclaurine levels in human urine after the administration of a throat lozenge containing Nandina domestica fruit.[Pubmed: 28801989]
Higenamine is a key component of traditional Chinese herbal medicine. The fruit of Nandina domestica (which contains this component) is available as an ingredient in the so-called Nanten-nodo-ame throat lozenge found on the Japanese market, which is an over-the-counter pharmaceutical and is easy to purchase for Japanese athletes. However, higenamine is a non-selective β2-agonist, which is exemplified in the prohibited list of the World Anti-Doping Agency (WADA). Therefore, some have raised a concern regarding the potential cause of increased unintentional higenamine doping cases in the Asian region. This study aimed to investigate components of throat lozenges and develop a mass-spectrometry method for the quantification of higenamine and coclaurine in human urine. Moreover, a population study of Japanese subjects (n = 246) and an excretion study (n = 4) of the corresponding throat-lozenge recipients were performed to test the applicability of the current reporting threshold (i.e., 10 ng/mL) of higenamine set by WADA. The estimates of higenamine and coclaurine were 2.2 ± 0.1 μg/drop (mean of n = 12) and 0.5 ± 0.01 μg/drop (mean of n = 12), respectively. The maximum concentrations of higenamine and coclaurine were 0.2-0.4 and 0.3-1.0 ng/mL, respectively, at 10-12 h after administration of higenamine (nine drops); however, the concentrations in all four volunteers did not reach the positivity criterion of 10 ng/mL. No higenamine and coclaurine could be detected in the Japanese subjects. Therefore, there is no risk of detecting unintentional higenamine doping when the WADA reporting threshold is used.
Pediatric Nandina domestica ingestions reported to poison centers.[Pubmed: 28421827]
Nandina domestica is grown as an ornamental plant in the United States but has also been reported as an invasive plant in a number of states. Parts of the plant, particularly the berries, contain cyanogenic glycosides that convert to hydrogen cyanide when ingested. This investigation characterized N. domestica ingestions involving patients of age 5 years and less reported to Texas poison centers during 2000-2015. There were 875 total N. domestica ingestions. A seasonal pattern was observed with the highest proportion of ingestions occurring in March (18.5%) and April (14.7%). The patients were male in 55.0% of the cases; 40.8% of the patients were of age 1 and 37.0% of age 2. Berries were specifically mentioned in 709 ingestions, of which 57.3% involved one berry and 28.5% an unknown number of berries. The ingestion occurred at the patient's own residence in 92.9% of the cases, and the patient was managed on site in 97.0%. The most frequently reported clinical effects were vomiting (3.7%), abdominal pain (1.0%), diarrhea (0.9%), and nausea (0.7%). In conclusion, N. domestica ingestions among young children generally do not result in serious outcomes and can be managed successfully outside of a healthcare facility.
Complete genome sequences of two highly divergent Japanese isolates of Plantago asiatica mosaic virus.[Pubmed: 27743255]
Plantago asiatica mosaic virus (PlAMV) is a member of the genus Potexvirus and has an exceptionally wide host range. It causes severe damage to lilies. Here we report on the complete nucleotide sequences of two new Japanese PlAMV isolates, one from the eudicot weed Viola grypoceras (PlAMV-Vi), and the other from the eudicot shrub Nandina domestica Thunb. (PlAMV-NJ). Their genomes contain five open reading frames (ORFs), which is characteristic of potexviruses. Surprisingly, the isolates showed only 76.0-78.0 % sequence identity with each other and with other PlAMV isolates, including isolates from Japanese lily and American nandina. Amino acid alignments of the replicase coding region encoded by ORF1 showed that the regions between the methyltransferase and helicase domains were less conserved than other regions, with several insertions and/or deletions. Phylogenetic analyses of the full-length nucleotide sequences revealed a moderate correlation between phylogenetic clustering and the original host plants of the PlAMV isolates. This study revealed the presence of two highly divergent PlAMV isolates in Japan.
The complete chloroplast genome of Sinopodophyllum hexandrum (Berberidaceae).[Pubmed: 26704891]
The complete chloroplast (cp) genome of the Sinopodophyllum hexandrum (Berberidaceae) was determined in this study. The circular genome is 157,940 bp in size, and comprises a pair of inverted repeat (IR) regions of 26,077 bp each, a large single-copy (LSC) region of 86,460 bp and a small single-copy (SSC) region of 19,326 bp. The GC content of the whole cp genome was 38.5%. A total of 133 genes were identified, including 88 protein-coding genes, 37 tRNA genes and eight rRNA genes. The whole cp genome consists of 114 unique genes, and 19 genes are duplicated in the IR regions. The phylogenetic analysis revealed that S. hexandrum is closely related to Nandina domestica within the family Berberidaceae.
Caffeoyl glucosides from Nandina domestica inhibit LPS-induced endothelial inflammatory responses.[Pubmed: 26410076]
Endothelial dysfunction is a key pathological feature of many inflammatory diseases, including sepsis. In the present study, a new caffeoyl glucoside (1) and two known caffeoylated compounds (2 and 3) were isolated from the fruits of Nandina domestica Thunb. (Berberidaceae). The compounds were investigated for their effects against lipopolysaccharide (LPS)-mediated endothelial inflammatory responses. At 20 μM, 1 and 2 inhibited LPS-induced hyperpermeability, adhesion, and migration of leukocytes across a human endothelial cell monolayer in a dose-dependent manner suggesting that 1 and 2 may serve as potential scaffolds for the development of therapeutic agents to treat vascular inflammatory disorders.
Chemical composition and antioxidant activities of the essential oil from Nandina domestica fruits.[Pubmed: 26199150]
The chemical composition and antioxidant activities of the essential oil from Nandina domestica fruits were studied for the first time. Twenty-two compounds, representing 82.79% of the oil, were identified from the oil. The major compounds were 3-hexen-1-ol (12.9%), linalool (12.3%), 2-methoxy-4-vinylphenol (9.9%), oleic acid (8.0%), furfural (5.8%) and 2,6-di-tert-butyl-4-methylphenol (5.7%). The antioxidant activities of the oil were evaluated using reducing power, metal chelating ability and scavenging capacity against 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2'-azinobis-3-ethylbenzthiazoline-6-sulfonate (ABTS) and superoxide anion free radical. The oil exhibited significant antioxidant activities.


