Pandanus amaryllifolius
Pandanus amaryllifolius
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Pandanus amaryllifolius
- Cat.No. Product Name CAS Number COA
-
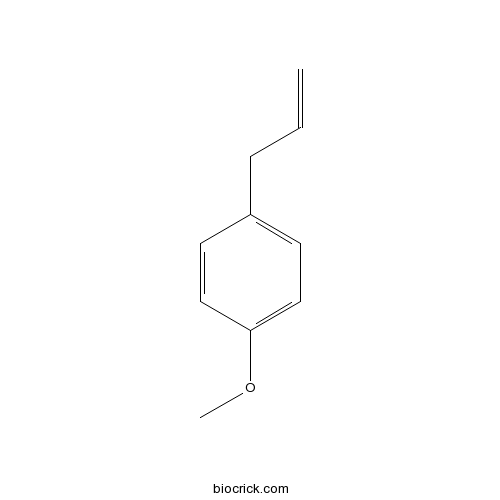
BCC8674
4-Allylanisole140-67-0
Instructions
-
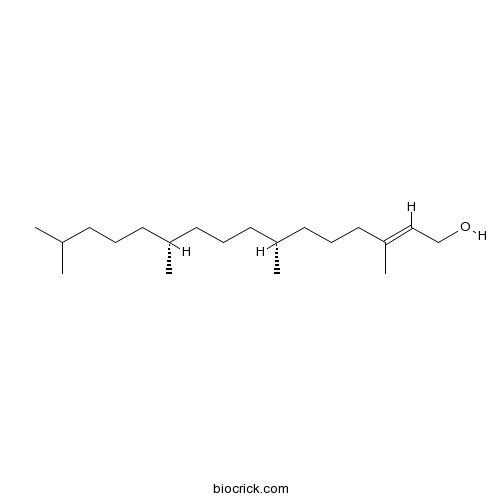
BCN1673
Phytol150-86-7
Instructions
p-Coumaroyl Malate Derivatives of the Pandanus amaryllifolius Leaf and Their Isomerization.[Pubmed: 29199224]
A novel p-coumaroyl dimethyl malate (1) was isolated from the Pandanus amaryllifolius leaf in addition to three known analogs of p-coumaroyl dimethyl malate (2-4), and their structures were elucidated by analysis of the spectroscopic data. The p-coumaroyl malate derivatives were isolated as a mixture of E and Z isomers. To determine the cause of isomerization, the p-coumaroyl malate isolated in this study was synthesized. We concluded that the Z isomer might be an artifact generated from the E isomer through purification steps.
Alkaloids from Pandanus amaryllifolius: Isolation and Their Plausible Biosynthetic Formation.[Pubmed: 26461164]
Pandanus amaryllifolius Roxb. (Pandanaceae) is used as a flavor and in folk medicine in Southeast Asia. The ethanolic crude extract of the aerial parts of P. amaryllifolius exhibited antioxidant, antibiofilm, and anti-inflammatory activities in previous studies. In the current investigation, the purification of the ethanolic extract yielded nine new compounds, including N-acetylnorpandamarilactonines A (1) and B (2); pandalizines A (3) and B (4); pandanmenyamine (5); pandamarilactones 2 (6) and 3 (7), and 5(E)-pandamarilactonine-32 (8); and pandalactonine (9). The isolated alkaloids, with either a γ-alkylidene-α,β-unsaturated-γ-lactone or γ-alkylidene-α,β-unsaturated-γ-lactam system, can be classified into five skeletons including norpandamarilactonine, indolizinone, pandanamine, pandamarilactone, and pandamarilactonine. A plausible biosynthetic route toward 1-5, 7, and 9 is proposed.
First Total Synthesis of Pandamarine.[Pubmed: 26126132]
The first total synthesis of pandamarine, an alkaloid isolated from Pandanus amaryllifolius is reported. The key step of this extremely short (six steps in total) and protecting group-free synthesis is a highly efficient cascade reaction sequence initiated by the photooxidation of an easily accessible and symmetric difuran precursor.
Antihyperglycemic effects of Pandanus amaryllifolius Roxb. leaf extract.[Pubmed: 25709220]
Diabetes mellitus is one of the leading chronic diseases worldwide. In patients with poor glycemic control, high blood glucose level may lead to other life-threatening complications. Pandanus amaryllifolius Roxb. (PA) leaves are used in traditional medicine for the treatment of diabetes.
Carotenoid-cleavage activities of crude enzymes from Pandanous amryllifolius.[Pubmed: 25408328]
Carotenoid degradation products, known as norisoprenoids, are aroma-impact compounds in several plants. Pandan wangi is a common name of the shrub Pandanus amaryllifolius. The genus name 'Pandanus' is derived from the Indonesian name of the tree, pandan. In Indonesia, the leaves from the plant are used for several purposes, e.g., as natural colorants and flavor, and as traditional treatments. The aim of this study was to determine the cleavage of β-carotene and β-apo-8'-carotenal by carotenoid-cleavage enzymes isolated from pandan leaves, to investigate dependencies of the enzymatic activities on temperature and pH, to determine the enzymatic reaction products by using Headspace Solid Phase Microextraction Gas Chromatography/Mass Spectrophotometry (HS-SPME GC/MS), and to investigate the influence of heat treatment and addition of crude enzyme on formation of norisoprenoids. Crude enzymes from pandan leaves showed higher activity against β-carotene than β-apo-8'-carotenal. The optimum temperature of crude enzymes was 70°, while the optimum pH value was 6. We identified β-ionone as the major volatile reaction product from the incubations of two different carotenoid substrates, β-carotene and β-apo-8'-carotenal. Several treatments, e.g., heat treatment and addition of crude enzymes in pandan leaves contributed to the norisoprenoid content. Our findings revealed that the crude enzymes from pandan leaves with carotenoid-cleavage activity might provide a potential application, especially for biocatalysis, in natural-flavor industry.
[Chemical components from essential oil of Pandanus amaryllifolius leaves].[Pubmed: 25345137]
To analyze the chemical compositions of Pandanus amaryllifolius leaves essential oil extracted by steam distillation.
Remediation of nutrient-rich waters using the terrestrial plant, Pandanus amaryllifolius Roxb.[Pubmed: 25076532]
Effective control of eutrophication is generally established through the reduction of nutrient loading into waterways and water bodies. An economically viable and ecologically sustainable approach to nutrient pollution control could involve the integration of retention ponds, wetlands and greenways into water management systems. Plants not only play an invaluable role in the assimilation and removal of nutrients, but they also support fauna richness and can be aesthetically pleasing. Pandanus amaryllifolius, a tropical terrestrial plant, was found to establish well in hydrophytic conditions and was highly effective in remediating high nutrient levels in an aquatic environment showing 100% removal of NO3(-)-N up to 200 mg/L in 14 days. Phosphate uptake by the plant was less efficient with 64% of the PO4(-)-P removed at the maximum concentration of 100 mg/L at the end of 6 weeks. With its high NO3(-)-N and PO4(3-)-P removal efficiency, P. amaryllifolius depleted the nutrient-rich media and markedly contained the natural colonization of algae. The impediment of algal growth led to improvements in the water quality with significant decreases in turbidity, pH and electrical conductivity. In addition, the plants did not show stress symptoms when grown in high nutrient levels as shown by the changes in their biomass, total soluble proteins and chlorophyll accumulation as well as photochemical efficiency. Thus, P. amaryllifolius is a potential candidate for the mitigation of nutrient pollution in phytoremediation systems in the tropics as the plant requires low maintenance, is tolerant to the natural variability of weather conditions and fluctuating hydro-periods, and exhibit good nutrient removal capabilities.
Characterization of pR18, a novel rolling-circle replication plasmid from Lactobacillus plantarum.[Pubmed: 24785193]
Lactobacillus plantarum PA18, a strain originally isolated from the leaves of Pandanus amaryllifolius, contains a pR18 plasmid. The pR18 plasmid is a 3211bp circular molecule with a G+C content of 35.8%. Nucleotide sequence analysis revealed two putative open reading frames, ORF1 and ORF2, in which ORF2 was predicted (317 amino acids) to be a replication protein and shared 99% similarity with the Rep proteins of pLR1, pLD1, pC30il, and pLP2000, which belong to the RCR pC194/pUB110 family. Sequence analysis also indicated that ORF1 was predicted to encode linA, an enzyme that enzymatically inactivates lincomycin. The result of Southern hybridization and mung bean nuclease treatment confirmed that pR18 replicated via the RCR mechanism. Phylogenetic tree analysis of pR18 plasmid proteins suggested that horizontal transfer of antibiotic resistance determinants without genes encoding mobilization has not only occurred between Bacillus and Lactobacillus but also between unrelated bacteria. Understanding this type of transfer could possibly play a key role in facilitating the study of the origin and evolution of lactobacillus plasmids. Quantitative PCR showed that the relative copy number of pR18 was approximately 39 copies per chromosome equivalent.


