Phellinus igniarius
Phellinus igniarius
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Phellinus igniarius
- Cat.No. Product Name CAS Number COA
-
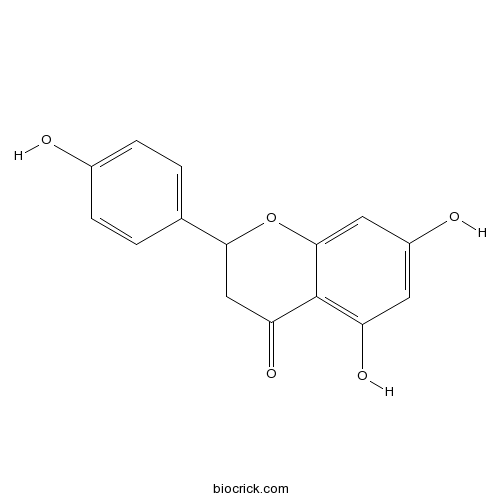
BCN1209
Eriodictyol552-58-9
Instructions

-
BCN9061
(±)-Naringenin67604-48-2
Instructions
-
BCN6309
Coumarin91-64-5
Instructions

Anti-Inflammatory Activity of Chemical Constituents Isolated from the Willow Bracket Medicinal Mushroom Phellinus igniarius (Agaricomycetes).[Pubmed: 29773004]
Nuclear factor-κB (NF-κB) has an important role in immune response and inflammation. Phellinus igniarius (known as Sang huang in traditional Chinese medicine) has various pharmacologic effects. In this study, 19 chemical constituents (1-19) were isolated from the fruiting bodies of Ph. igniarius. Their structures were elucidated based on data from nuclear magnetic resonance spectroscopy and comparison with the literature. Overall, 3 compounds (2, 7, and 11) from this species were reported, to our knowledge for the first time. Two compounds (4 and 15) from the genus Phellinus and 9 compounds (1, 3, 5, 8-10, and 12-14) from the Hymenochaetaceae family were also reported for the first time. An NF-κB luciferase reporter assay of these compounds was carried out in tumor necrosis factor-α-induced HeLa cells-again, to our knowledge for the first time. Among them, compounds 5 and 7 showed moderate inhibition of NF-κB, with fold values of 0.48 ± 0.02 and 0.55 ± 0.09, respectively, in HeLa cells at 100 μmol/L. These results suggest that some of the ingredients from Ph. igniarius could be developed into antiinflammatory agents for use in clinical applications.
In Vitro Evaluation of the Antiviral Activity of Some Mushrooms from Turkey.[Pubmed: 29717666]
Despite considerable recent work to reveal different features of mushrooms species, the few studies of antiviral activities are inadequate and therefore further studies are required. Morchella conica, M. esculenta, Terfezia boudieri, Pleurotus ostreatus, Tricholoma anatolicum, Fomes fomentarius, Laetiporus sulphureus, Phellinus igniarius, Porodaedalea pini, and Pyrofomes demidoffii from Turkey were investigated to reveal their in vitro cytotoxic and anti-herpes simplex virus 1 (HSV-1) activities. The crude methanol extracts (MEs) and aqueous extracts (AEs) of fungal species and acyclovir (ACV) were used. Various dilutions were used to assess the cytotoxic effects of fungal species (50-0.10 mg/mL) and ACV (500-0.98 μg/mL) on uninfected Vero cells. Maximum nontoxic concentrations were determined for all extracts and ACV by comparing the optical densities of their cell controls. The concentration providing 50% protection against the cytopathic effect caused by the virus, extracts, and ACV (EC50) was calculated, and the half-maximal cytotoxic concentration (CC50) and the selectivity index (SI) were determined, the latter as the ratio of CC50 to EC50. While the AEs of F. fomentarius (EC50, 11.22 mg/mL; SI > 4.46), Ph. igniarius (EC50, 9.71 mg/mL; SI > 5.15), and P. pini (EC50, 7.16 mg/mL; SI > 6.98) showed considerable antiherpetic activity, MEs and AEs of the other fungal species did not showed any effects. The EC50 and SI of ACV were determined as 0.20 μg/mL and 3085, respectively. The results demonstrate that F. fomentarius, Ph. igniarius, and P. pini have important anti-HSV-1 activity.
Medicinal mushroom Phellinus igniarius induced cell apoptosis in gastric cancer SGC-7901 through a mitochondria-dependent pathway.[Pubmed: 29549725]
None
Anti-diabetic activity of a polyphenol-rich extract from Phellinus igniarius in KK-Ay mice with spontaneous type 2 diabetes mellitus.[Pubmed: 29271444]
The present study investigated the anti-diabetic activity and potential mechanisms of the polyphenol rich extract from Phellinus igniarius (PI-PRE) in vitro and in vivo. Four main phenolic compounds of PI-PRE were purified and identified as 7,8-dihydroxycoumarin, 3,4-dihydroxybenzalacetone, 7,3'-dihydroxy-5'-methoxyisoflavone and inoscavin C by the off-line semipreparative liquid chromatography-nuclear magnetic resonance protocol. In vitro, PI-PRE stimulated GLUT4 translocation by 2.34-fold and increased glucose uptake by 1.73-fold in L6 cells. However, the selective AMP-activated protein kinase (AMPK) inhibitor, compound C, completely reversed the PI-PRE-induced GLUT4 translocation. In vivo, KK-Ay mice treated with PI-PRE for four weeks had lower fasting blood glucose levels, as well as other blood-lipid indexes, compared with the vehicle control group. Mechanistic studies showed that the expressions of p-AMPKα and GLUT4 were significantly increased by treatment with PI-PRE in L6 cells. In KK-Ay mice, the expression of p-AMPKα was enhanced in the liver and skeletal muscle, and the expression of GLUT4 was increased in skeletal muscle. These findings suggest that PI-PRE possesses potential anti-diabetic effects including improving glucose tolerance, reducing hyperglycemia, and normalizing insulin levels. These effects are partly due to the activation of GLUT4 translocation via the modulation of the AMPK pathway.
Chemical constituents from the fruiting bodies of Phellinus igniarius.[Pubmed: 29232973]
A new tirucallane-type triterpenoid igniarine (1), and four known compounds meshimakobnol A (2), meshimakobnol B (3), ergosterol (4) and ergosterol peroxide (5), were purified from the methanol extracts of the fruiting bodies of Phellinus igniarius (DC. ex Fr.) Quél. The structure of 1 was elucidated using a combination of 1D and 2D NMR techniques and HR-ESI-MS analyses. In addition, the isolated compounds were examined for their cytotoxicity against several tumour cell lines and part of the tested compounds demonstrated weak cytotoxicity.
Cytotoxic Activities of Fractions of the Willow Bracket Medicinal Mushroom, Phellinus igniarius (Agaricomycetes), and the Induction of Cell Cycle Arrest and Apoptosis in MGC-803 Cells.[Pubmed: 29199565]
Phellinus igniarius, a perennial medicinal mushroom, has been used in China and other countries of East Asia for the treatment of various diseases, including cancer. The purpose of this study is to evaluate the cytotoxic activities of different fractions of an ethanol extract from Ph. igniarius and to elucidate a possible antitumor mechanism. An ethanol extract of Ph. igniarius was partitioned into a petroleum ether fraction, an ethyl acetate fraction (EAF), an n-butanol fraction, and a water-soluble fraction. The cytotoxic activity of all the fractions was initially screened in a brine shrimp lethality test, then evaluated by the Cell Counting Kit-8 assay against 5 human tumor cell lines: MGC-803, BEL-7402, HeLa, MCF-7, and HCT-116. The cell cycle distribution induced by EAF on MGC-803 cells was analyzed by flow cytometry with propidium iodide staining, and apoptosis was determined using flow cytometry with Annexin V/propidium iodide staining. The results of the brine shrimp lethality test and the Cell Counting Kit-8 assay showed that EAF was the most active fraction, displaying strong inhibitory activity against the MGC-803, BEL-7402, and MCF-7 cancer cell lines. Flow cytometry analysis indicated that EAF could induce S-phase cell cycle arrest in MGC-803 cells and cause apoptosis in a concentration-dependent manner. This study demonstrated that EAF, as the most active fraction of Ph. igniarius, exerted antitumor activity by inducing MGC-803 cell apoptosis via S-phase cell cycle arrest.
Growth-Inhibitory and Immunomodulatory Activities of Wild Mushrooms from North-Central British Columbia (Canada).[Pubmed: 29199559]
Wild mushrooms, especially from North America, have not been systematically explored for their medicinal properties. Here we report screening for the growth-inhibitory and immunomodulatory activities of 12 species collected from multiple locations in north-central British Columbia, Canada. Mushrooms were characterized using morphology and DNA sequencing, followed by chemical extraction into 4 fractions using 80% ethanol, 50% methanol, water, and 5% sodium hydroxide. Growth-inhibitory, immunostimulatory, and anti-inflammatory activities of 5 mushrooms (Leucocybe connata, Trichaptum abietinum, Hydnellum sp., Gyromitra esculenta, and Hericium coralloides) are reported here, to our knowledge for the first time. Growth-inhibitory effects were assessed using the cytotoxic MTT assay. Immunostimulatory activity was assessed by tumor necrosis factor-α production in Raw 264.7 macrophages, whereas anti-inflammatory activity was assessed based on the inhibition of lipopolysaccharide-induced tumor necrosis factor-α production. The ethanol and aqueous extracts of Hydnellum sp. were potent growth inhibitors, with a half-maximal inhibitory concentration of 0.6 mg/mL. All 5 fungi displayed strong immunostimulatory activity, whereas only L. connata and T. abietinum showed strong anti-inflammatory activity. For the 7 other fungi investigated, which included well-known medicinal species such as Inonotus obliquus, Phellinus igniarius, and Ganoderma applanatum, the remarkable similarities in the biological activities reported here, and by others for specimens collected elsewhere, suggest that mushrooms can produce similar metabolites regardless of their habitat or ecosystem. This is to our knowledge the first study to explore wild mushrooms from British Columbia for biological activities that are relevant to cancer, and the results provide an initial framework for the selection of mushroom species with the potential for discovery of novel anticancer compounds.
Hispolon suppresses metastasis via autophagic degradation of cathepsin S in cervical cancer cells.[Pubmed: 28981104]
Hispolon, a phenolic compound isolated from Phellinus igniarius, induces apoptosis and anti-tumor effects in cancers. However, the molecular mechanism involved in hispolon-mediated tumor-suppressing activities observed in cervical cancer is poorly characterized. Here, we demonstrated that treatment with hispolon inhibited cell metastasis in two cervical cancer cell lines. In addition, the downregulation of the lysosomal protease Cathepsin S (CTSS) was critical for hispolon-mediated suppression of tumor cell metastasis in both in vitro and in vivo models. Moreover, hispolon induced autophagy, which increased LC3 conversion and acidic vesicular organelle formation. Mechanistically, hispolon inhibited the cell motility of cervical cells through the extracellular signal-regulated kinase (ERK) pathway, and blocking of the ERK pathway reversed autophagy-mediated cell motility and CTSS inhibition. Our results indicate that autophagy is essential for decreasing CTSS activity to inhibit tumor metastasis by hispolon treatment in cervical cancer; this finding provides a new perspective on molecular regulation.
[Chemical constituents from Phellinus igniarius and their anti-tumor activity in vitro].[Pubmed: 28920346]
Eleven compounds were isolated and purified from Phellinus igniarius by column chromatography on silica gel, Sephedax LH-20, RP-8, MCI and preparative TLC. Their structures were identified as 3α-hydroxyfriedel-2-one (1), 3-hydroxyfriedel-3-en-2-one (2), ergosta-4, 6, 8 (14), 22-tetraen-3-one (3), ergosterol peroxide (4), uracil (5), uridine (6), 4-(3, 4-dihydroxyphenyl)-3-butene-2-one (7), protocatechualdehyde (8), inotilone (9), inoscavinA (10) and phellibaumin E (11), respectively, on the basis of NMR and MS data analysis. Among them, compounds 1, 2, 5, and 6 were firstly obtained from this genus. In vitro cytotoxic activity of compounds 1-11 was screened by Cell Titer-GLo Reagent, on 41 human tumor cell strains and 2 hamster normal cell strains via high-throughput screening. Compounds 2-4 exhibit significant cytotoxic activity against NOMO-1 and SKM-1 acute myeloid leukemia cell lines, and compounds 2 and 3 showed good selectivity to NOMO-1 with IC₅₀ values of 0.795 5, 1.828 μmol•L-1and SKM-1 with IC₅₀ values of higher than 10 μmol•L-1. Compound 7 showed remarkable antitumor activities against H526 Human lung cancer cell line, DU145 prostate cancer cell line and HEL erythroleukemia cell line with IC₅₀ values of 0.533 4, 1.885, 1.057 μmol•L⁻¹, respectively. Other compounds had no or weak antitumor effect. In addition, all compounds had no significant effect on hamster normal cell lines CHL and CHO with IC₅₀ values of higher than 10 μmol•L⁻¹, which showed that all compounds had no toxic effect on normal cells.


