Phlomis umbrosa
Phlomis umbrosa
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Phlomis umbrosa
- Cat.No. Product Name CAS Number COA
-
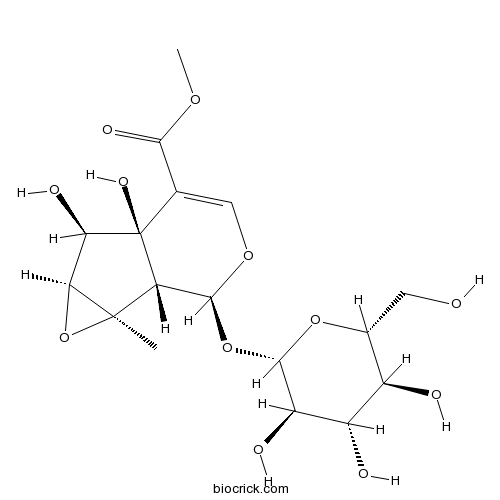
BCN6051
Sesamoside117479-87-5
Instructions
-
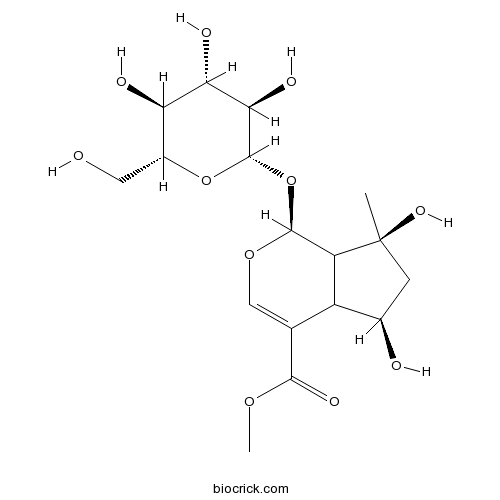
BCN4187
Shanzhiside methylester64421-28-9
Instructions
-
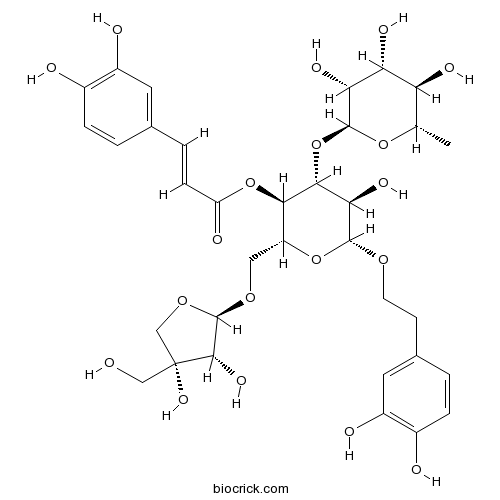
BCN1205
Forsythoside B81525-13-5
Instructions
Safety evaluation of Astragalus extract mixture HT042 and its constituent herbs in Sprague-Dawley rats.[Pubmed: 28732808]
Astragalus extract mixture HT042 is a combination of three standardized extracts from Astragalus membranaceus root, Eleutherococcus senticosus stem, and Phlomis umbrosa root, which has proven to stimulate children's height growth.
Effects of Phlomis umbrosa Root on Longitudinal Bone Growth Rate in Adolescent Female Rats.[Pubmed: 27070559]
This study aimed to investigate the effects of Phlomis umbrosa root on bone growth and growth mediators in rats. Female adolescent rats were administered P. umbrosa extract, recombinant human growth hormone or vehicle for 10 days. Tetracycline was injected intraperitoneally to produce a glowing fluorescence band on the newly formed bone on day 8, and 5-bromo-2'-deoxyuridine was injected to label proliferating chondrocytes on days 8-10. To assess possible endocrine or autocrine/paracrine mechanisms, we evaluated insulin-like growth factor-1 (IGF-1), insulin-like growth factor binding protein-3 (IGFBP-3) or bone morphogenetic protein-2 (BMP-2) in response to P. umbrosa administration in either growth plate or serum. Oral administration of P. umbrosa significantly increased longitudinal bone growth rate, height of hypertrophic zone and chondrocyte proliferation of the proximal tibial growth plate. P. umbrosa also increased serum IGFBP-3 levels and upregulated the expressions of IGF-1 and BMP-2 in growth plate. In conclusion, P. umbrosa increases longitudinal bone growth rate by stimulating proliferation and hypertrophy of chondrocyte with the increment of circulating IGFBP-3. Regarding the immunohistochemical study, the effect of P. umbrosa may also be attributable to upregulation of local IGF-1 and BMP-2 expressions in the growth plate, which can be considered as a GH dependent autocrine/paracrine pathway.
Effect of HT042, herbal formula, on longitudinal bone growth in spontaneous dwarf rats.[Pubmed: 24169467]
HT042 is a new herbal prescription consisting of Astragalus membranaceus, Phlomis umbrosa and Eleutherococcus senticosus, which are used in Korean medicine to stimulate growth in children. We investigated the effects of HT042 on the body weight, longitudinal bone growth, and bone length in spontaneous dwarf rats (SDR). Male and female SDRs were divided into three groups: the control group (DW, 10 mL/kg/day), the recombinant human GH group (rhGH; 500 µg/kg/day), and the HT042 (100 mg/kg/day) group. Each group received the respective treatments for 10 days. Body weight was measured on day 10 of treatment. On day 8, tetracycline (20 mg/kg) was injected intraperitoneally into all individuals to form a fluorescent band on the newly synthesized bone. On day 10, femur and tibia lengths were measured using PIXImus. Body weight, longitudinal bone growth, and bone length were not affected in the HT042 group. In contrast, the rhGH group showed significantly increased body weight, longitudinal bone growth, and bone length. In conclusion, HT042 does not act through a GH-like effect to promote longitudinal bone growth.
Two new dammarane-type glycosides from Phlomis umbrosa.[Pubmed: 23972014]
Two new dammarane-type glycosides, phlomisumbroside A (1) and phlomisumbroside B (2), together with 15 known compounds (3-17) were isolated from the leaves of Phlomis umbrosa Turcz. Their structures were established by the spectroscopic methods including 2D NMR techniques.
Skeletal growth and IGF levels in rats after HT042 treatment.[Pubmed: 22388791]
HT042, a new herbal prescription consisting of Astragalus membranaceus, Phlomis umbrosa and Eleutherococcus senticosus, is used in traditional Korean medicine to stimulate growth in children. This study was conducted to investigate the effects of HT042 on skeletal growth, insulin-like growth factor-1 (IGF-1) and insulin-like growth factor binding protein-3 (IGFBP-3) levels, and oestrogenic activity in female rats. Female Sprague-Dawley rats were divided into control, recombinant human growth hormone (rhGH; 20 µg/kg/day), and HT042 (100 mg/kg/day) groups and treated for 3 weeks. Axial skeletal growth, femur length, and growth plate length were measured every 3 weeks. The serum IGF-1 and IGFBP-3 levels were analysed. Moreover, the oestrogenic activity of the herbal extracts in the immature and ovariectomized rats was tested. The nose-anus, nose-tail, femur and growth-plate lengths were increased significantly in the HT042 group. Both IGF-1 and IGFBP-3 were highly expressed in the hypertrophic zone of the growth plate. The serum IGF-1 levels were increased. Moreover, HT042 had no uterotrophic effects in the rats. Consequently, HT042 promoted longitudinal bone growth by stimulating cell proliferation in the epiphyseal plate and inducing the expression of IGF-1 without an oestrogenic response. HT042 may be helpful in stimulating growth in children with short stature.
The effect of herbal extract (EstroG-100) on pre-, peri- and post-menopausal women: a randomized double-blind, placebo-controlled study.[Pubmed: 21887807]
This clinical research study was designed to evaluate the efficacy of a new herbal product, EstroG-100, containing a mixture of standardized extracts of Cynanchum wilfordii, Phlomis umbrosa and Angelica gigas, on menopausal symptoms. This randomized double-blind, placebo-controlled trial was performed for 12 weeks with 64 pre-, peri- and postmenopausal White Hispanic, White non-Hispanic and African American women who were randomly allocated to either the EstroG-100 group (n = 31) or the placebo group (n = 33). Primary end-points were the mean change in scores of the Kupperman menopause index (KMI) that evaluates 11 symptoms, and the mean change in scores of vaginal dryness. The mean KMI score was significantly reduced in the EstroG-100 group from 29.5 ± 7.4 at baseline to 11.3 ± 5.8 (p < 0.01) compared with change of the placebo group (29.2 ± 6.6 at baseline vs 23.7 ± 7.7 at week 12). The constituting symptoms of vasomotor, paresthesia, insomnia, nervousness, melancholia, vertigo, fatigue and rheumatic pain were significantly improved in the EstroG-100 group in comparison with the placebo group (p < 0.05). Statistically significant improvement in vaginal dryness in the EstroG-100 group was also observed compared with that of the placebo group (p < 0.05). In conclusion, EstroG-100 significantly improved the menopausal symptoms of pre-, peri- and post-menopausal women without weight gain or any serious side effects.


