Physalis alkekengi var. franchetii
Physalis alkekengi var. franchetii
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Physalis alkekengi var. franchetii
- Cat.No. Product Name CAS Number COA
-
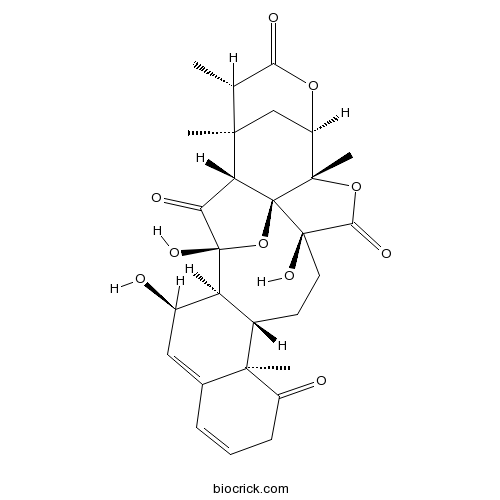
BCN2312
Physalin L113146-74-0
Instructions
-
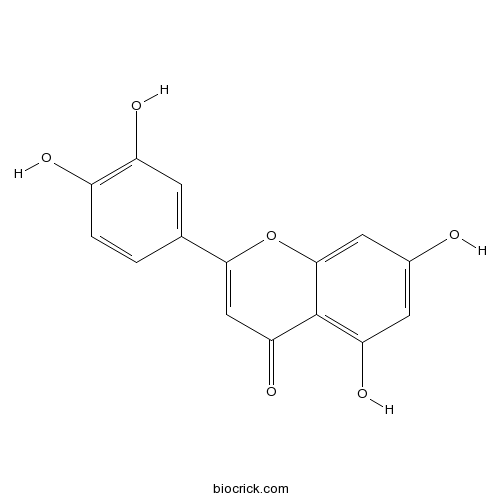
BCN5600
Luteolin491-70-3
Instructions
-
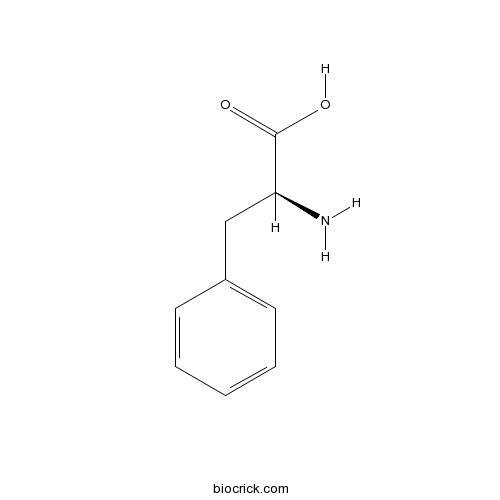
BCN3818
L-Phenylalanine63-91-2
Instructions
Physakengose G induces apoptosis via EGFR/mTOR signaling and inhibits autophagic flux in human osteosarcoma cells.[Pubmed: 29655686]
Physakengose G (PG) is a new compound first isolated from Physalis alkekengi var. franchetii, an anticarcinogenic traditional Chinese medicine. PG has shown promising anti-tumor effects, but its underlying mechanisms remain unknown.
Identification of absorbed constituents and in vivo metabolites in rats after oral administration of Physalis alkekengi var. franchetii by ultra-high-pressure liquid chromatography quadrupole time-of-flight mass spectrometry.[Pubmed: 29055030]
The calyces of Physalis alkekengi var. franchetii (Chinese Lantern, JDL) are well-known as traditional Chinese medicine owing to its various therapeutic effects. However, the bioactive constituents responsible for the pharmacological effects of JDL and their metabolites in vivo are still unclear to date. In this paper, an ultra-high-pressure liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (UHPLC/Q-TOF-MS/MS) method was established to identify absorbed constituents and in vivo metabolites in rat biological fluids after oral administration of JDL. Based on the proposed strategy, 33 compounds were observed in dosed rat biosamples. Twelve of 33 compounds were indicated as prototype components of JDL, and 21 compounds were predicted to be metabolites of JDL. Finally, the metabolic pathways were proposed, which were glucuronidation, sulfation, methylation and dehydroxylation for flavonoid constituents and sulfonation and hydroxylation for physalin consitituents. This is the first systematic study on the absorbed constituents and metabolic profiling of JDL and will provide a useful template for screening and characterizing the ingredients and metabolites of traditional Chinese medicine.
Physakengoses K-Q, seven new sucrose esters from Physalis alkekengi var. franchetii.[Pubmed: 28779658]
Seven sucrose esters, physakengoses K-Q (1-7) were isolated from the aerial parts of Physalis alkekengi var. franchetii. Their structures were elucidated on the basis of extensive spectroscopic analyses and chemical methods. These new compounds were tested for their antimicrobial abilities against Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa and Escherichia coli. Among the isolated sucrose esters, compounds 1-5 showed potent antibacterial activity with MIC values ranging from 2.16 to 12.76 μg/mL.
LC-MS/MS method for simultaneous determination of flavonoids and physalins in rat plasma: Application to pharmacokinetic study after oral administration of Physalis alkekengi var. franchetii (Chinese lantern) extract.[Pubmed: 28273367]
None
Anti-hyperglycemic activity of polysaccharides from calyx of Physalis alkekengi var. franchetii Makino on alloxan-induced mice.[Pubmed: 28238911]
The present study was undertaken to investigate the hypoglycemic effect of polysaccharides (PPSC) isolated from calyx of Physalis alkekengi var. franchetii Makino in alloxan-induced diabetic mice. Seven polysaccharide fractions were obtained through a DEAE-52 column and Sephadex G-100 gel column and they were the main composes of PPSC. The PPSC treatment could prevent the loss of body weight in diabetic mice and result in a decrease of fasting blood glucose (FBG) and glycated serum protein (GSP) and an increase of fasting serum insulin in a dose-dependent manner. The histopathological examination of pancreas revealed the ability of PPSC to protect and reverse β-cells from necrosis due destruction of alloxan in diabetic mice. Furthermore, oral PPSC upregulated the expression of PI3K, Akt and GLUT4 mRNA in skeletal muscles and adipose tissues. The results suggest that PPSC possess significant anti-diabetic activity, as evaluated using alloxanised diabetic mice model. Consequently, PPSC might be a promising candidate for the development of a new anti-diabetic agent.
Metabolic profiles of physalin A in rats using ultra-high performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry.[Pubmed: 28157662]
Physalin A, one of the major active components isolated from the calyces of Physalis alkekengi var. franchetii is considered to be a promising natural product due to its anti-inflammatory and excellent antitumor activities. Until now, only one paper is available from our group concerning identification of two sulfonate metabolites from rat feces after physalin A treatment. All the other researches related to physalin A were focused on its extraction, separation and biological activities. In this research, a rapid and reliable ultra-high-performance liquid chromatography/quadrupole time-of-flight mass spectrometry (UPLC/Q-TOF-MS/MS) method was developed and employed for the comprehensive study of the metabolism of physalin A in vivo for the first time. A total of 24 proposed metabolites were identified in plasma, bile, urine and feces of rats after oral administration of physalin A. The results indicated that sulfonation, reduction and hydroxylation were the major metabolic pathways of physalin A in vivo. Furthermore, this research provides scientific and reliable support for full understanding of the metabolism of physalin A and the results could help to elucidate the safety and efficacy of physalin A, as well as other physalins.


