Pieris formosa
Pieris formosa
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Pieris formosa
- Cat.No. Product Name CAS Number COA
-
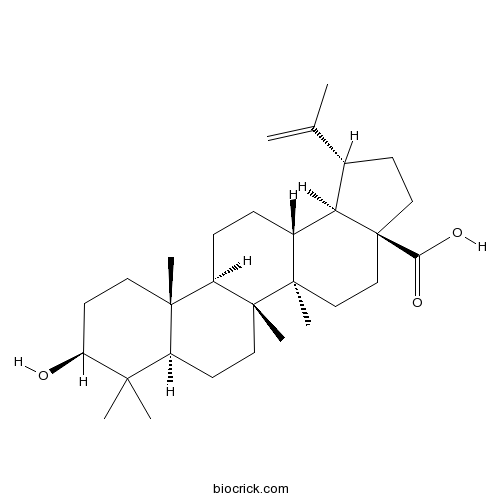
BCN5524
Betulinic acid472-15-1
Instructions
-
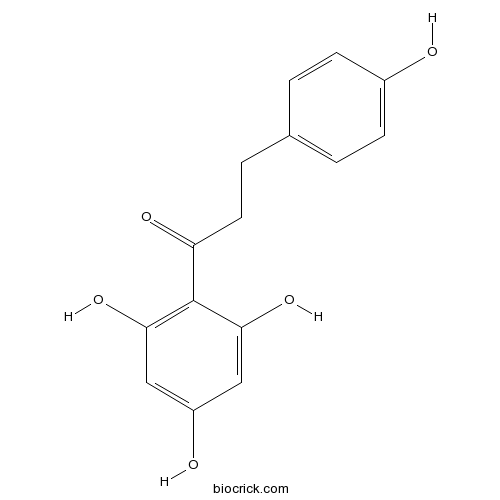
BCN4128
Phloretin60-82-2
Instructions
[Chemical constituents from roots of Pieris formosa and their bioactivity].[Pubmed: 29676095]
None
New Antifeedant Grayanane Diterpenoids from the Flowers of Pieris formosa.[Pubmed: 28858256]
None
Pierisketolide A and Pierisketones B and C, Three Diterpenes with an Unusual Carbon Skeleton from the Roots of Pieris formosa.[Pubmed: 28151673]
Pierisketolide A (1) and pierisketones B and C (2 and 3), three diterpenes with an unusual A-homo-B-nor-ent-kaurane carbon skeleton, were isolated from the roots of Pieris formosa. Their structures were characterized by a series of spectroscopic methods, X-ray diffraction, and electronic circular dichroism (ECD). Pierisketolide A (1) exhibited an analgesic effect with a 45% writhe inhibition rate at a dose of 10.0 mg/kg. The plausible biosynthetic pathways of 1-3 are proposed.
Pierisformotoxins A-D, polyesterified grayanane diterpenoids from Pieris formosa and their cAMP-decreasing activities.[Pubmed: 23776021]
Four highly acylated diterpenoids, designated as pierisformotoxins A-D (1-4, resp.), along with 26 known compounds, were isolated from the flowers of Pieris formosa. Among them, pierisformotoxins A and B (1 and 2, resp.) were new highly acylated grayanane diterpenoids, of which the five-membered ring A has undergone an oxidative cleavage between C(3) and C(4), followed by lactonization, to give rise to a five-membered lactone ring between C(3) and C(5), differing from the previously reported grayanane diterpenoids with a 5/7/6/5 ring system. Results of the cAMP-regulation-activity assay showed that pierisformotoxin C (3) at 10 μM (inhibitory ratio (IR): 10.1%) or 2 μM (9.8%), and pierisformotoxin B (2) at 50 μM (13.9%) significantly decreased the cAMP level in N1E-115 neuroblastoma cells (p<0.05).
Three new highly acylated 3,4-seco-grayanane diterpenoids from the fruits of Pieris formosa.[Pubmed: 21467681]
Phytochemical investigation on the fruits of Pieris formosa resulted in the isolation of three new highly acylated 3,4-seco-grayanane diterpenoids, pierisformotoxins E-G (1-3). Their structures were elucidated on the basis of extensive spectroscopic analysis, including 1D-, 2D-NMR, electrospray ionization-mass spectra (ESI-MS) and high resolution (HR)-MS.
Novel polyesterified 3,4-seco-grayanane diterpenoids as antifeedants from Pieris formosa.[Pubmed: 20405952]
Pieris formosa is a poisonous plant to livestock and is used as an insecticide in rural areas of China. Two novel polyesterified 3,4-seco-grayanane diterpenoids, pierisoids A and B (1 and 2), were isolated from its flowers and were identified by spectroscopic analysis and X-ray diffraction. Both compounds showed obvious antifeedant activity against cotton bollworm, indicating their toxic properties, suggesting a defensive role of polyesterified 3,4-seco-grayanane diterpenoids for P. formosa against herbivores.
New grayanol diterpenoid and new phenolic glucoside from the flowers of Pieris formosa.[Pubmed: 20390746]
A new grayanol diterpenoid, grayanotoxin XXII (1), and a new phenolic glucoside, benzyl 2-hydroxy-4-O-[beta-xylopyranosyl(1'' --> 6')-beta-glucopyranosyl]-benzoate (2), were isolated from the flowers of Pieris formosa. Their structures were determined on the basis of spectroscopic analysis and chemical methods.


