Pogonatherum crinitum
Pogonatherum crinitum
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Pogonatherum crinitum
- Cat.No. Product Name CAS Number COA
-
BCN6049
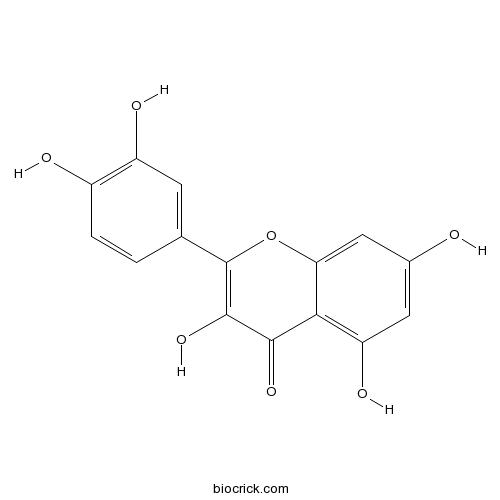
Quercetin117-39-5
Instructions
-
BCN2205
D-Mannitol69-65-8
Instructions

Phytoremediation of fluoride with garden ornamentals Nerium oleander, Portulaca oleracea, and Pogonatherum crinitum.[Pubmed: 28097483]
None
Phytoremediation of textile dyes and effluents: Current scenario and future prospects.[Pubmed: 26386310]
Phytoremediation has emerged as a green, passive, solar energy driven and cost effective approach for environmental cleanup when compared to physico-chemical and even other biological methods. Textile dyes and effluents are condemned as one of the worst polluters of our precious water bodies and soils. They are well known mutagenic, carcinogenic, allergic and cytotoxic agents posing threats to all life forms. Plant based treatment of textile dyes is relatively new and hitherto has remained an unexplored area of research. Use of macrophytes like Phragmites australis and Rheum rhabarbarum have shown efficient removal of Acid Orange 7 and sulfonated anthraquinones, respectively. Common garden and ornamental plants namely Aster amellus, Portulaca grandiflora, Zinnia angustifolia, Petunia grandiflora, Glandularia pulchella, many ferns and aquatic plants have also been advocated for their dye degradation potential. Plant tissue cultures like suspension cells of Blumea malcolmii and Nopalea cochenillifera, hairy roots of Brassica juncea and Tagetes patula and whole plants of several other species have confirmed their role in dye degradation. Plants' oxidoreductases such as lignin peroxidase, laccase, tyrosinase, azo reductase, veratryl alcohol oxidase, riboflavin reductase and dichlorophenolindophenol reductase are known as key biodegrading enzymes which break the complex structures of dyes. Schematic metabolic pathways of degradation of different dyes and their environmental fates have also been proposed. Degradation products of dyes and their fates of metabolism have been reported to be validated by UV-vis spectrophotometry, high performance liquid chromatography, high performance thin layer chromatography, Fourier Transform Infrared Spectroscopy, gas chromatograph-mass spectroscopy and several other analytical tools. Constructed wetlands and various pilots scale reactors were developed independently using the plants of P. australis, Portulaca grandiflora, G. pulchella, Typha domingensis, Pogonatherum crinitum and Alternanthera philoxeroides. The developed phytoreactors gave noteworthy treatments, and significant reductions in biological oxygen demand, chemical oxygen demand, American Dye Manufacturers Institute color removal value, total organic carbon, total dissolved solids, total suspended solids, turbidity and conductivity of the dye effluents after phytoremediation. Metabolites of dyes and effluents have been assayed for phytotoxicity, cytotoxicity, genotoxicity and animal toxicity and were proved to be non/less toxic than untreated compounds. Effective strategies to handle fluctuating dye load and hydraulics for in situ treatment needs scientific attention. Future studies on development of transgenic plants for efficacious phytodegradation of textile dyes should be focused.
Treatment of textile effluent in a developed phytoreactor with immobilized bacterial augmentation and subsequent toxicity studies on Etheostoma olmstedi fish.[Pubmed: 25464312]
A static hydroponic bioreactor using nursery grown plants of Pogonatherum crinitum along with immobilized Bacillus pumilus cells was developed for the treatment of textile wastewater. Independent reactors with plants and immobilized cells were also kept for performance and efficacy evaluation. The effluent samples characterized before and after their treatment showed that the plant-bacterial consortium reactor was more efficient than those of individual plant and bacterium reactors. COD, BOD, ADMI, conductivity, turbidity, TDS and TSS of the textile effluent was found to be reduced by 78, 70, 93, 4, 90, 13 and 70% respectively within 12 d by the consortial set. HPTLC analysis revealed the transformation of the textile effluent to new products. The phytotoxicity study on Phaeseolus mungo and Sorghum vulgare seeds showed reduced toxicity of treated effluents. The animal toxicity study performed on Etheostoma olmstedi fishes showed the toxic nature of untreated effluent giving extreme stress to fishes leading to death. Histology of fish gills exposed to treated effluent was found to be less affected. The oxidative stress related enzymes like superoxide dismutase and catalase were found to show decreased activities and less lipid peroxidation in fishes exposed to treated effluent.
Lead-induced nitric oxide generation plays a critical role in lead uptake by Pogonatherum crinitum root cells.[Pubmed: 22904111]
The effects of lead (Pb) on endogenous nitric oxide (NO) generation, the role of NO in Pb uptake and the origin of Pb-induced NO production in Pogonatherum crinitum root cells were evaluated. Pb treatment induced rapid NO generation, showing that Pb exposure triggered endogenous NO signaling of the cells. Pre-treatment of the cells with the NO-specific scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline -1-oxyl-3-oxide (cPTIO) not only abolished the Pb-triggered NO burst but also reduced Pb contents of the cells. Moreover, Pb exposure enhanced nitrate reductase (NR) activity of the cells. The NR inhibitors tungstate and glutamine not only suppressed the Pb-enhanced NR activities but also reduced the Pb-triggered NO generation. Pre-treatment of the cells with tungstate and glutamine suppressed Pb accumulation and the suppression could be restored by application of exogenous NO via its donors sodium nitroprusside (SNP) and S-nitrosoglutathione (GSNO). Together, our results indicated that Pb exposure enhanced NR activity and triggered the NO burst of P. crinitum root cells. Furthermore, the data demonstrated that NR was responsible for the Pb-triggered NO burst and that NR-mediated NO generation played a critical role in Pb uptake by P. crinitum root cells. Thus, our results suggest a potential strategy for controlling Pb uptake by plants by targeting NR as a source of Pb-triggered NO production.
Flavonoids with iNOS inhibitory activity from Pogonatherum crinitum.[Pubmed: 18448292]
Pogonatherum crinitum has long been used as a folk remedy for the treatment of many inflammatory diseases in Taiwan, and till now there is still no report concerning its active principles as well as their pharmacological studies. That prompted us to investigate the bioactive constituents of Pogonatherum crinitum. Two novel chemical entities, luteolin 6-C-beta-boivinopyranoside (1) and 6-trans-(2''-O-alpha-rhamnopyranosyl)ethenyl-5,7,3',4'-tetrahydroxyflavone (2), along with luteolin (3), kaempferol (4), luteolin 6-C-beta-fucopyranoside (5), kaempferol 3-O-alpha-L-rhamnopyranoside (6), luteolin 6-C-beta-glucopyranoside (7), rutin (8) and kaempferol 3-O-rutinoside (9) were isolated from this plant, and identified by spectroscopic analysis. The effect of these compounds on the inhibition of NO production in LPS-activated macrophages was further evaluated. All these compounds inhibited NO production in activated RAW 264.7 cells to various degrees without affecting the cellular viability. Among the compounds examined, both compounds 1 and 2 suppressed LPS-induced NO production, with E(max) values of 99.51+/-0.23% and 92.41+/-3.22%, respectively. The most potent compounds, 3 and 4, inhibited NO production with IC(50) values of 10.41+/-0.02 microM and 10.61+/-0.44 microM, respectively. These effects were attributed to suppression of mRNA expression of inducible NO synthase (iNOS). Our results clearly demonstrated that these naturally occurring iNOS inhibitors may be beneficial to the treatment of inflammatory diseases associated with overproduction of NO, which provides an explanation, at least a part, for the anti-inflammatory property of Pogonatherum crinitum.


