Pouzolzia zeylanica var. microphylla
Pouzolzia zeylanica var. microphylla
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Pouzolzia zeylanica var. microphylla
- Cat.No. Product Name CAS Number COA
-
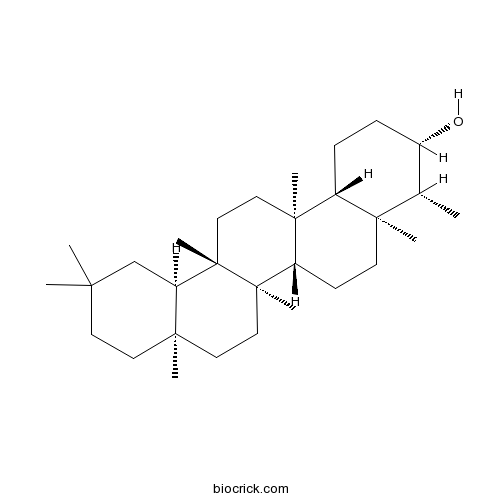
BCN1104
Epifriedelanol16844-71-6
Instructions
-
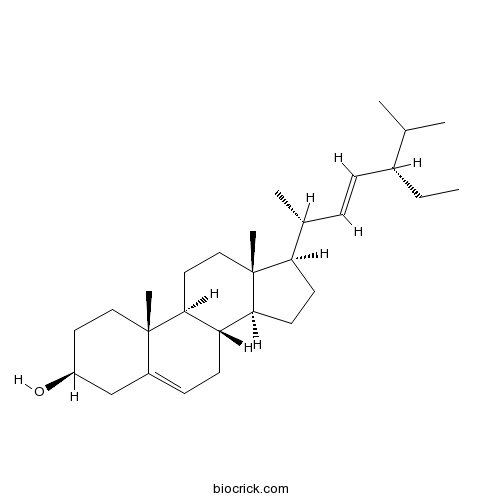
BCN4376
Stigmasterol83-48-7
Instructions
Norlignans from Pouzolzia zeylanica var. microphylla and their nitric oxide inhibitory activity.[Pubmed: 26067593]
Five new compounds, pouzolignan F [4-hydroxy-3-(3,5-dihydroxyphenyl)-2-[bis(4-hydroxy-3-methoxyphenyl)methyl]butyl acetate] (1), pouzolignan G [4-hydroxy-3-(3,5-dihydroxyphenyl)-2-[(4-hydroxy-3,5-dimethoxyphenyl)(4-hydroxy-3-methoxyphenyl)methyl]butyl acetate] (2), pouzolignan H [1,4-dihydroxy-3-(3,5-dihydroxyphenyl)-2-[bis(4-hydroxy-3-methoxyphenyl)methyl]butane] (3), pouzolignan I [1,4-dihydroxy-3-(3,5-dihydroxyphenyl)-2-[(4-hydroxy-3,5-dime thoxyphenyl)-(4-hydroxy-3-methoxyphenyl)methyl]butane] (4), and pouzolignan J [1,4-dihydroxy-3-(3,5-dihydroxyphenyl) -2-[(3,4,5-trimethoxyphenyl)(4-hydroxy-3-methoxyphenyl)methyl]butane] (5), along with two known compounds, indolyl-3-carboxylic acid (6) and uracil (7), were isolated from the aerial parts of Pouzolzia zeylanica (L.) Benn. var. microphylla (Wedd.) W.T.Wang. The structures of these compounds were characterized based on spectroscopic methods, including IR, NMR ((1)H-(1)H COSY, HSQC, HMBC, and NOESY), and HR-ESI/TOF-MS experiments. All the new norlignans were assayed for inhibitory activity against lipopolysaccharide (LPS)-induced nitric oxide (NO) production in mouse peritoneal macrophages.


