Ranunculus ternatus
Ranunculus ternatus
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Ranunculus ternatus
- Cat.No. Product Name CAS Number COA
-
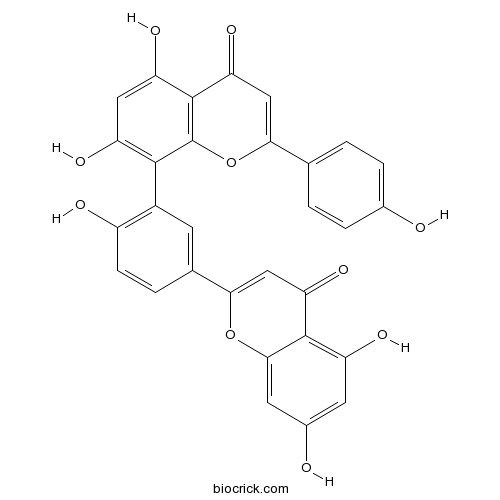
BCN6283
Amentoflavone1617-53-4
Instructions
-
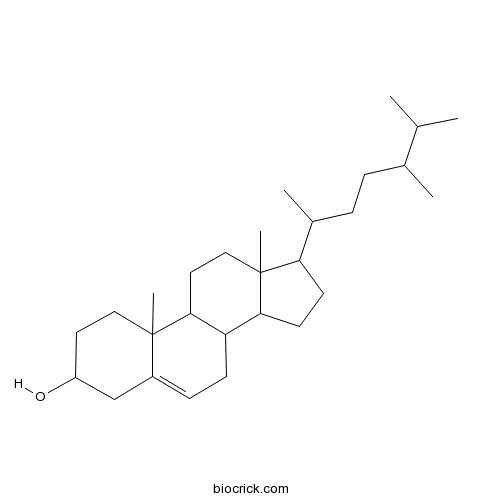
BCN3181
Campesterol474-62-4
Instructions
-
BCN2653
Phillygenin487-39-8
Instructions

-
BCN5661
Bilobetin521-32-4
Instructions

-
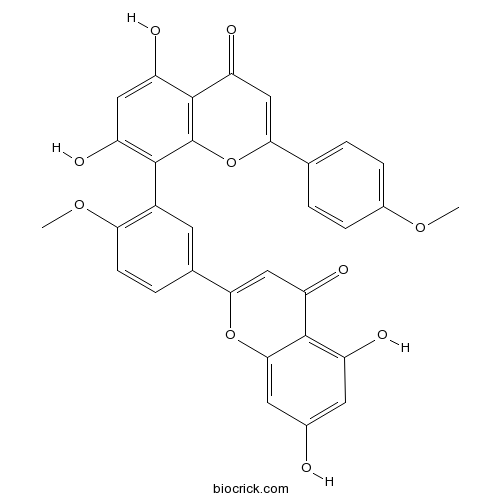
BCN2320
Isoginkgetin548-19-6
Instructions
-
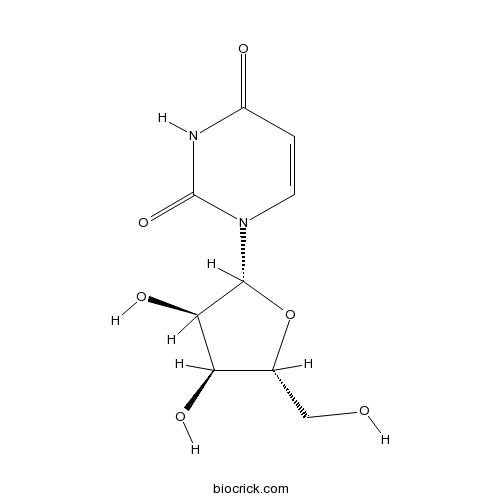
BCN4090
Uridine58-96-8
Instructions
-
BCN4540
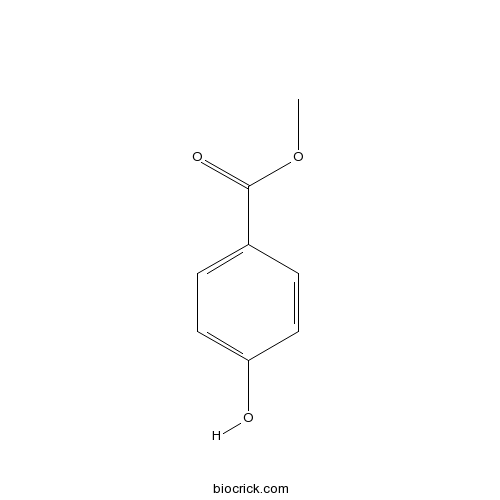
Methyl 4-hydroxybenzoate99-76-3
Instructions
New heterocyclic compounds from Ranunculus ternatus Thunb.[Pubmed: 28727990]
Five new heterocyclic compounds, 5-α-d-fructofuranosylmethyl-furfural (1), 5-β-d-fructofuranosylmethyl-furfural (2), 5-β-d-fructopyranosylmethyl-furfural (3), 4-(2-((2S-2,3-dihydroxypropoxy)methyl)-5-formyl-1H-pyrrol-1-yl)butanoic acid (4), and 3S,4S-4,5,8-trihydroxy-3-(prop-1-en-2-yl)isochroman-1-one (5), were obtained from the root of Ranunculus ternatus Thunb., which is a traditional Chinese anti-tuberculosis medicine. Their structures were elucidated by UV, IR, HRESIMS, NMR data, and the comparison of experimental and calculated electronic circular dichroism (ECD) spectra. Notably, compounds 1-3 are rarely occurring furfural fructosides in natural sources. These heterocyclic compounds could be further studied for the synthetic chemists and pharmacologists due to the source and structural properties.
Naturally occurring hybrids derived from γ-amino acids and sugars with potential tail to tail ether-bonds.[Pubmed: 27166276]
The basic substances of life include various amino acids and sugars. To search such molecules is the precondition to understand the essential nature. Here we reported four unprecedented hybrids of γ-amino acids and sugars from the roots of Ranunculus ternatus, which possess potential tail to tail ether-connected (6,6-ether-connected) modes in the sugar moiety. The structures of these hybrids were elucidated by extensive analyses of spectra and calculated electronic circular dichroism (ECD) method.
[Analysis of flavonoids and components of stems & leaves of Ranunculus ternatus Thunb. using ultra performance liquid chromatography-quadrupole tandem time of flight mass spectrometry].[Pubmed: 24392620]
In order to explore the applications of liquid chromatography-mass spectrometry (LC-MS) technology in the rapid identification of components of Chinese herbal medicines and natural products, reference flavones were used as precursors, and the stems and leaves of medicinal plant Ranunculus ternatus Thunb. were used as the objects. Ultra performance liquid chromatography with a diode array detector-electrospray ionization quadrupole tandem time of flight mass spectrometry (UPLC/DAD-ESI/Q-TOF MS) was also used to analyse the characteristics of flavonoid homologues and flavonoid isomers. The results showed that ultraviolet (UV) absorption and MS/MS spectra of C-glycosyl were distinct from O-glycosyl. There was also a correlation between glycosidation positions and the retention times, MS/MS fragments and their relative abundances. When applied it to analyse the alcohol extract of stems and leaves of Ranunculus ternatus Thunb. and their acid hydrolysis solution, 22 flavonol glycosides and 3 flavonoid aglycones were identified. The method is simple, convenient and exercisable.
Chemical constituents from the roots of Ranunculus ternatus and their inhibitory effects on Mycobacterium tuberculosis.[Pubmed: 24071991]
Two new benzophenones, methyl (R)-3-[2-(3,4-dihydroxybenzoyl)-4,5-dihydroxyphenyl]-2-hydroxypropanoate (1) and n-butyl (R)-3-[2-(3,4-dihydroxybenzoyl)-4,5-dihydroxyphenyl]-2-hydroxypropanoate (2), were isolated from the roots of Ranunculus ternatus along with the two known compounds vanillic acid (3) and gallic acid (4). Their structures were elucidated by physical and spectroscopic analysis. In addition, compound 1 exhibited obvious activity against tuberculosis, while the activity of a 1:1 mixture of compound 1 plus 4 is better than that of 1 alone.
[Rapid determination of fatty acids in Ranunculus ternatus Thunb by microwave-ultrasonic synergistic one-step extraction-derivatization and gas chromatography-mass spectrometry].[Pubmed: 23785996]
A rapid and simple microwave-ultrasonic synergistic one-step extraction-derivatization (MUED) method and gas chromatography-mass spectrometry was established for the determination of low content fatty acids (FAs) profile in Ranunculus ternatus Thunb. The critical experimental parameters for MUED method were optimized with response surface methodology by taking the chromatographic peak areas of total FAs as a major response index. The best technological parameters were determined as 5.0 g of Ranunculus ternatus Thunb. powder, 50.0 mL of n-hexane, 500 W of microwave power, 50 degree C of reaction temperature, 0.30 g of catalyst (KOH), 4.0 mL of derivatization reagent (methanol) and the time of extraction-derivatization of 8 min. The contents of individual FAs were quantified by internal standard method. The results showed that the chromatographic peak areas of the total FAs and the total unsaturated FAs contents obtained with MUED were (3.327 +/- 0.023) x 10(7) (n = 3) and (13.59 +/- 0.30) mg/g (n = 3) respectively. They were markedly higher than those obtained by the conventional method which were (2.410 +/- 0.036) x 10(7) (n = 3) and (12.05 +/- 0.34) mg/g (n = 3) respectively. The MUED method simplified the complicated sample handling steps, shortened the sample preparation time, reduced the cost of analysis, and improved the extraction and derivatization efficiency of the lipids, especially weakened the oxidization and decomposition of the unsaturated FAs. The simplicity, speed and practicability suggest the proposed method has significant potential for the determination of lowcontent FAs in herbal medicines.
Ternatusine A, a new pyrrole derivative with an epoxyoxepino ring from Ranunculus ternatus.[Pubmed: 23557518]
Ternatusine A (1), a novel alkaloid with an unprecedented epoxyoxepino[4,5-c] pyrrole ring, was isolated from the roots of Ranunculus ternatus Thunb. Its unusual structure, including its absolute stereochemistry, was determined using UV, IR, HRESIMS, and 1D and 2D NMR data and through comparison of the experimental and calculated electronic circular dichroism (ECD) spectra. A possible biosynthetic pathway for ternatusine A was postulated.
[Studies on chemical constituents of Ranunculus ternatus].[Pubmed: 18619350]
To study the active constituents of Ranunculus ternatus.
Two new indolopyridoquinazoline alkaloidal glycosides from Ranunculus ternatus.[Pubmed: 17666858]
Two new indolopyridoquinazoline alkaloidal glycosides, 11-O-beta-D-glucopyranosyl rutaecarpine (ternatoside C) and 11-O-alpha-L-rhamnosyl-(1-->6)-beta-D-glucopyranosyl rutaecarpine (ternatoside D) were isolated from the roots of Ranunculus ternatus. Their structures were determined on the basis of spectroscopic and chemical methods.
[Determination of fatty acids and organic acids in Ranunculus ternatus Thunb using GC-MS].[Pubmed: 17058970]
The determination of fatty acids and organic acids in Chinese medicinal plant Ranunculus ternatus Thunb using GC-MS was studied. The Ranunculus ternatus Thunb from Henan province was cut into less than 20 mesh pieces, then extracted by petroleum ether or ether in refluxing and esteried, and finally was determined using GC-MS. The results show that there are 23 kinds of organic compounds in the Chinese medicinal plant Ranunculus ternatus Thunb from Henan, among which 15 kinds of fatty acids were identified, including myristic acid, palmitic acid, stearic acid, oleic acid, linolenic acid, eicosanoic acid, docosanoic acid etc. The unsaturated fatty acids and oleic acid account for 58.19% and 35.68% of the total organic compounds respectively. The kinds of fatty acid in petroleum ether extract and ether extract are the same.


