Rauvolfia verticillata
Rauvolfia verticillata
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Rauvolfia verticillata
- Cat.No. Product Name CAS Number COA
-
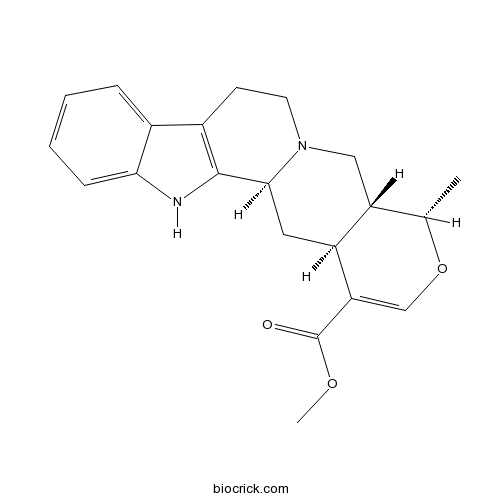
BCN5208
Robinin301-19-9
Instructions

-
BCN5577
Ajmalicine483-04-5
Instructions
-
BCN4960
Reserpine50-55-5
Instructions

Reserpine Inhibit the JB6 P+ Cell Transformation Through Epigenetic Reactivation of Nrf2-Mediated Anti-oxidative Stress Pathway.[Pubmed: 26988984]
Nuclear factor erythroid-2 related factor 2 (Nrf2) is a crucial transcription factor that regulates the expression of defensive antioxidants and detoxification enzymes in cells. In a previous study, we showed that expression of the Nrf2 gene is regulated by an epigenetic modification. Rauvolfia verticillata, a traditional Chinese herbal medicine widely used in China, possesses anticancer and antioxidant effects. In this study, we investigated how Nrf2 is epigenetically regulated by reserpine, the main active component in R. verticillata, in mouse skin epidermal JB6 P+ cells. Reserpine induced ARE (antioxidant response element)-luciferase activity in HepG2-C8 cells. Accordingly, in JB6 P+ cells, it upregulated the mRNA and protein levels of Nrf2 and its downstream target genes heme oxygenase-1 (HO-1) and
Hexacyclic monoterpenoid indole alkaloids from Rauvolfia verticillata.[Pubmed: 26474672]
Five new hexacyclic monoterpenoid indole alkaloids, rauvovertine A (1), 17-epi-rauvovertine A (2), rauvovertine B (3), 17-epi-rauvovertine B (4), and rauvovertine C (5) together with 17 known analogues were isolated from the stems of Rauvolfia verticillata. Compounds 1/2 and 3/4 were obtained as C-17 epimeric mixtures due to rapid hemiacetal tautomerism in solution. The structures of 1-5 were established by spectroscopic analysis and with the aid of molecular modeling. The new alkaloids were evaluated for their cytotoxicity in vitro against human tumor HL-60, SMMC-7721, A-549, MCF-7, and SW-480 cell lines.
Pectic polysaccharides extracted from Rauvolfia verticillata (Lour.) Baill. var. hainanensis Tsiang increase LκB-α expression and ameliorate ulcerative colitis.[Pubmed: 25997255]
The therapeutic potential of pectic polysaccharides extracted from Rauvolfia verticillata (Lour.) Baill. var. hainanensis Tsiang in ulcerative colitis were investigated. This study showed that pectic polysaccharides extracted from Rauvolfia verticillata (Lour.) Baill. var. hainanensis Tsiang ameliorated ulcerative colitis and were proposed to exhibit anti-inflammatory effects via increased expression of IκB-α proteins and suppressing NF-αB translocation.
Indole alkaloids from leaves and twigs of Rauvolfia verticillata.[Pubmed: 24266393]
Seven new indole alkaloids, rauverines A-G (1-7), and 19 known indole alkaloids were isolated from the leaves and twigs of Rauvolfia verticillata. All compounds showed no cytotoxicity against five human cancer cell lines, human myeloid leukemia (HL-60), hepatocellular carcinoma (SMMC-7721), lung cancer (A-549), breast cancer (MCF-7), and colon cancer (SW480) cells.
Determination of indole alkaloids and highly volatile compounds in Rauvolfia verticillata by HPLC-UV and GC-MS.[Pubmed: 23212134]
Rauvolfia verticillata (Lour.) Baill. (also called Luofumu in Chinese) is commonly used in traditional Chinese medicine for lowering blood pressure. In this study, a high-performance liquid chromatography assay using ultraviolet detection is described for the simultaneous measurement of the five bioactive indole alkaloids (sarpagine, yohimbine, ajmaline, ajmalicine and reserpine) in Rauvolfia. The detection of all five compounds was conducted at 280 nm. In quantitative analysis, the five compounds showed good regressions (R(2) > 0.9988) within the test ranges, and the recovery of the method was in the range of 90.4-101.4%. In addition, a simple gas chromatography mass method using a DB-1 silica capillary column (30 m × 0.25 mm i.d., 0.25 µm) is described for the identification of the highly volatile compounds in Rauvolfia. In qualitative analysis, more than 39 compounds were assayed and identified using the mass function and the National Institute of Standards and Technology database search system. The results demonstrated that the combination of quantitative and qualitative analyses offered an efficient way to evaluate the quality and consistency of Rauvolfia verticillata.
[Chemical constituents of Rauvolfia verticillata].[Pubmed: 22919724]
The study on the Rauvolfia verticillata (Lour.) Baill., which belongs to Apocynaceae, was carried out to look for its chemical constituents and pharmacological activity. The isolation and purification were performed by chromatography on silica gel, Sephadex LH-20 and ODS (octadecyl silane) open column. The structures of obtained compounds were elucidated on the basis of physicochemical properties and spectral analysis. Three indole alkaloids and one acridone alkaloid were isolated from chloroform layer extract and identified as ajmalicine B (1), sandwicine (2), raunescine (3) and 7-hydroxynoracronycine (4) separately. Ajmalicine B (1) is a new compound belonging to indole alkaloid. Compound 4 as an acridone alkaloid was a new type compound isolated from Rauvolfia genus for the first time. We also did some biological activity research on the new type compound (4) to explore other pharmacological activities in addition to antihypertensive activity.
Tryptophan decarboxylase plays an important role in ajmalicine biosynthesis in Rauvolfia verticillata.[Pubmed: 22331368]
Tryptophan decarboxylase (TDC) converts tryptophan into tryptamine that is the indole moiety of ajmalicine. The full-length cDNA of Rauvolfia verticillata (RvTDC) was 1,772 bps that contained a 1,500-bp ORF encoding a 499-amino-acid polypeptide. Recombinant 55.5 kDa RvTDC converted tryptophan into tryptamine. The K (m) of RvTDC for tryptophan was 2.89 mM, higher than those reported in other TIAs-producing plants. It demonstrated that RvTDC had lower affinity to tryptophan than other plant TDCs. The K (m) of RvTDC was also much higher than that of strictosidine synthase and strictosidine glucosidase in Rauvolfia. This suggested that TDC might be the committed-step enzyme involved in ajmalicine biosynthesis in R. verticillata. The expression of RvTDC was slightly upregulated by MeJA; the five MEP pathway genes and SGD showed no positive response to MeJA; and STR was sharply downregulated by MeJA. MeJA-treated hairy roots produced higher level of ajmalicine (0.270 mg g(-1) DW) than the EtOH control (0.183 mg g(-1) DW). Highest RvTDC expression level was detected in hairy root, about respectively 11, 19, 65, and 109-fold higher than in bark, young leaf, old leaf, and root. Highest ajmalicine content was also found in hairy root (0.249 mg g(-1) DW) followed by in bark (0.161 mg g(-1) DW) and young leaf (0.130 mg g(-1) DW), and least in root (0.014 mg g(-1) DW). Generally, the expression level of RvTDC was positively consistent with the accumulation of ajmalicine. Therefore, it could be deduced that TDC might be the key enzyme involved in ajmalicine biosynthesis in Rauvolfia.
A new 1-deoxy-D-xylulose 5-phosphate reductoisomerase gene encoding the committed-step enzyme in the MEP pathway from Rauvolfia verticillata.[Pubmed: 17542498]
1-Deoxy-D-xylulose 5-phosphate (DXP) reductoisomerase (DXR; EC 1.1.1.267) catalyzes a committed step of the methylerythritol phosphate (MEP) pathway for the biosynthesis of pharmaceutical terpenoid indole alkaloid (TIA) precursors. The full-length cDNA sequence was cloned and characterized from a TIA-producing species, Rauvolfia verticillata, using rapid amplification of cDNA ends (RACE) technique. The new cDNA was named as RvDXR and submitted to GenBank to be assigned with an accession number (DQ779286). The full-length cDNA of RvDXR was 1804 bp containing a 1425 bp open reading frame (ORF) encoding a polypeptide of 474 amino acids with a calculated molecular mass of 51.3 kDa and an isoelectric point of 5.88. Comparative and bioinformatic analyses revealed that RvDXR showed extensive homology with DXRs from other plant species and contained a conserved transit peptide for plastids, an extended Pro-rich region and a highly conserved NADPH-binding motif in its N-terminal region owned by all plant DXRs. The phylogenetic analysis revealed that DXRs had two groups including a plant and bacterial group; RvDXR belonged to angiosperm DXRs that were obtained from Synechocystis through gene transfer according to the phylogenetic analysis. The structural modeling of RvDXR showed that RvDXR had the typical V-shaped structure of DXR proteins. The tissue expression pattern analysis indicated that RvDXR expressed in all tissues including roots, stems, leaves, fruits and followers but at different levels. The lowest transcription level was observed in followers and the highest transcription was found in fruits of R. verticillata; the transcription level of RvDXR was a little higher in roots and stems than in leaves. The cloning and characterization of RvDXR will be helpful to understand more about the role of DXR involved in R. verticillata TIA biosynthesis at the molecular level and provides a candidate gene for metabolic engineering of the TIAs pathway in R. verticillata.


