Rhododendron micranthum
Rhododendron micranthum
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Rhododendron micranthum
- Cat.No. Product Name CAS Number COA
-
BCN1136
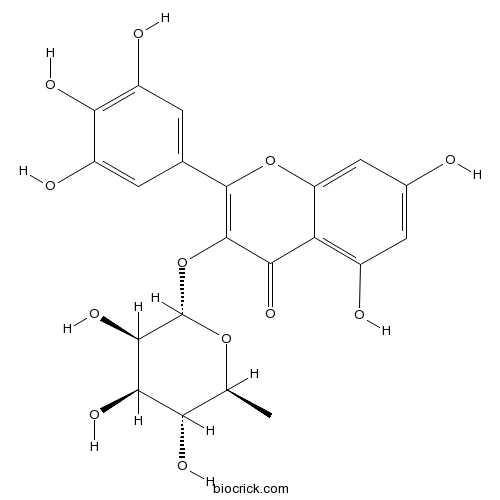
Myricitrin17912-87-7
Instructions
Grayanane and leucothane diterpenoids from the leaves of Rhododendron micranthum.[Pubmed: 26071839]
Eleven grayanane diterpenoids, 1-epi-grayanotoxin IV, 1-epi-grayanotoxin II, 6-deoxy-1-epi-grayanotoxin XVII, 6-deoxygrayanotoxin XVII, 16-acetylgrayanotoxin II, 3-oxograyanotoxin IX, 14-deoxygrayanotoxin VIII, 14-acetylisograyanotoxin II, rhodomicranols C-E, and a leucothane diterpenoid, rhodomicranol F, together with eleven known diterpenoids were isolated from leaves of Rhododendron micranthum. Their structures were elucidated by spectroscopic analyses, with the absolute configurations of 1-epi-grayanotoxin IV and rhodomicranol C determined by single-crystal X-ray diffraction with Cu Kα radiation, and the structures of 14-acetylisograyanotoxin II and known grayanotoxins IX and X confirmed by single-crystal X-ray diffraction. All twenty-three diterpenoids were evaluated for their in vitro immunomodulatory activities, and none showed significant immunomodulatory activities in a dose-dependent manner. In addition, they are non-toxic to the murine lymphocytes in the general cytotoxicity assay.
Micranthanone A, a new diterpene with an unprecedented carbon skeleton from Rhododendron micranthum.[Pubmed: 23745579]
A new diterpene with an unprecedented carbon skeleton, micranthanone A (1), two new grayanane diterpenoids bearing an unusual 5,6-(3,4-dihydroxylbenzylidene acetal) motif, rhodomicranols A (2) and B (3), and three known grayanane diterpenoids (4-6) were isolated from Rhododendron micranthum. Their structures were elucidated by spectroscopic analyses, calculated ECD, and single-crystal X-ray diffraction. The in vitro immunomodulatory activities of 1-6 were evaluated, and a plausible biogenetic pathway for 1 is proposed.
Identification and characterization of proanthocyanidins of 16 members of the Rhododendron genus (Ericaceae) by tandem LC-MS.[Pubmed: 22689627]
The proanthocyanidins of the leaves of 16 taxa of the Rhododendron genus (Ericaceae) [Rhododendron 'Catawbiense Grandiflorum', Rhododendron 'Cunningham's White', Rhododendron smirnowii Trautv., Rhododendron calophytum Franch., Rhododendron dichroanthum ssp. scyphocalyx (Balf. f. & Forrest ) Cowan, Rhododendron micranthum Turcz., Rhododendron praevernum Hutch., Rhododendron ungernii Trautv., Rhododendron kaempferi Planch., Rhododendron degronianum ssp. heptamerum var. hondoense (Nakai ) H. Hara, Rhododendron fortunei Lindl., Rhododendron ponticum L., Rhododendron galactinum Balf. f. ex Tagg., Rhododendron oreotrephes W. W. Sm., Rhododendron brachycarpum ssp. brachycarpum D. Don ex G. Don, and Rhododendron insigne Hemsl. & E. H. Wilson ] were investigated qualitatively by liquid chromatography-mass spectrometry in series. Twenty-nine dimeric proanthocyanidins based on (epi)catechin and (epi)gallocatechin were detected and characterized on the basis of their unique fragmentation pattern in the negative ion mode tandem mass spectrometry spectra. All of them were extracted for the first time from these sources, and ten of them were not reported previously in nature. The position of the galloyl residue was assigned on the basis of the retro-Diels-Alder fragmentation and the dehydrated retro-Diels-Alder fragmentation; it resulted from the loss of gallic acid as a neutral loss in the negative ion mode. Furthermore, four caffeoylquinic acids, six p-coumaroylquinic acids, epigallocatechin, gallocatechin, catechin, epicatechin, epigallocatechin gallate, catechin gallate, epicatechin gallate, gallocatechin gallate, two quercetin-O-hexosides, quercetin-O-galloyl-hexoside, quercetin-O-pentoside, quercetin-O-rhamnoside, quercetin-O-pentoside-O-hexoside, quercetin-O-rhamnoside-O-hexoside, quercetin-O-feruloyl-hexoside, quercetin-O-(p-hydroxy)benzoyl-hexoside, taxifolin-O-pentoside, myricetin-O-rhamnoside, two myricetin-O-pentosides, three myricetin-O-hexosides, and two myricetin-O-galloyl-hexosides were detected and shown to possess characteristic tandem mass spectrometry spectra and were tentatively assigned on the basis of their retention time.


