Scabiosa tschiliensis
Scabiosa tschiliensis
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Scabiosa tschiliensis
- Cat.No. Product Name CAS Number COA
-
BCN6219
Sweroside14215-86-2
Instructions
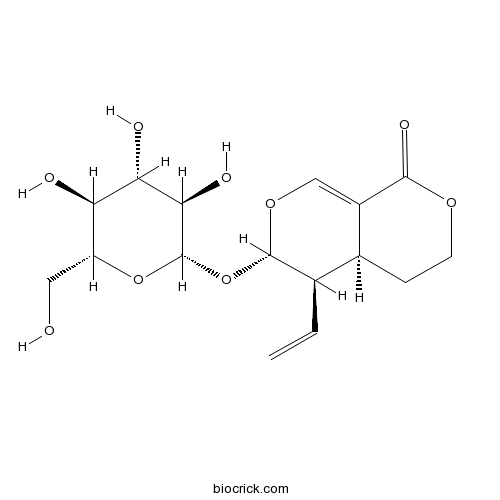
-
BCN5600
Luteolin491-70-3
Instructions
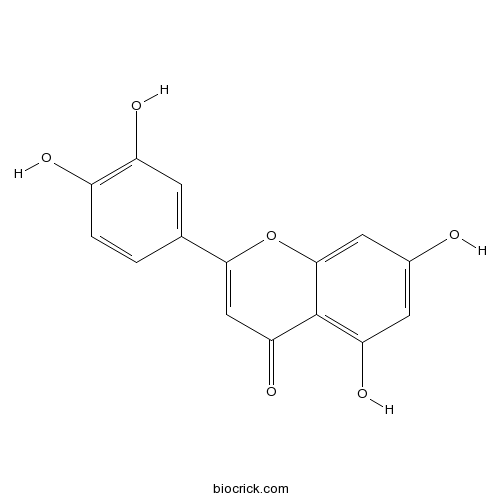
-
BCN5658
Apigenin520-36-5
Instructions

Facile synthesis of three bidesmosidic oleanolic acid saponins with strong inhibitory activity on pancreatic lipase.[Pubmed: 19463989]
The first synthesis of scabiosaponins E (1), F (2), and G (3), three new oleanolic acid saponins with strong inhibitory activity on pancreatic lipase isolated from the Chinese traditional medicinal herb Scabiosa tschiliensis, was efficiently achieved in an one-pot strategy under the combined use of glycosyl trichloroacetimidates and p-toluene 1-thioglycosides (STol) as donors.
New biologically active triterpenoid saponins from Scabiosa tschiliensis.[Pubmed: 15104490]
Eleven new triterpenoid saponins, scabiosaponins A-K (1-11), and hookerosides A (12) and B (13) were isolated from the whole plants of Scabiosa tschiliensis. The structures of the new compounds were established on the basis of extensive NMR (DEPT, DQF-COSY, HETCOR, TOCSY, HMQC, HMQC-TOCSY, HMBC, and NOESY) and MS studies coupled with chemical degradations. The biological activity of compounds 1-10, 12, and 13 and prosapogenin 1b were examined against pancreatic lipase. Scabiosaponins E, F, G, I (5, 6, 7, 9), hookerosides A, B (12, 13), and prosapogenin 1b all exhibited strong inhibition of pancreatic lipase in vitro.


