Securidaca inappendiculata
Securidaca inappendiculata
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Securidaca inappendiculata
- Cat.No. Product Name CAS Number COA
-
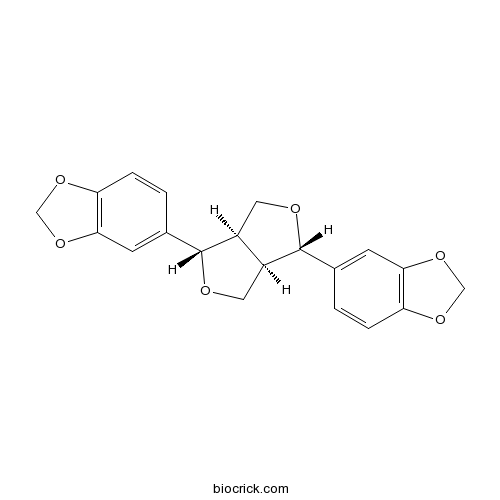
BCN4123
Sesamin607-80-7
Instructions
Methyl ferulic acid attenuates ethanol-induced hepatic steatosis by regulating AMPK and FoxO1 Pathways in Rats and L-02 cells.[Pubmed: 29940154]
Methyl ferulic acid (MFA) is a biologically active monomer extracted and purified from the Chinese herbal medicine Securidaca inappendiculata hasskarl. The previously studies showed that MFA improved acute liver injury induced by ethanol. However, the effect of MFA on ethanol-induced hepatic steatosis in alcoholic liver disease (ALD) still remains unclear. The current study was aimed at elucidating the effect of MFA on alcohol-induced hepatic steatosis and the underlying mechanisms. Human hepatocyte L-02 cells exposed to 200 mM ethanol for 24 h to simulate alcoholic steatosis in vitro. SD rats were fed a Lieber-DeCarli diet containing 5% (w/v) alcohol for 16 weeks to induce alcoholic liver disease in vivo. We examined the effect of MFA on ethanol-induced lipid deposition in L-02 cells and SD rats. The results showed that MFA reduced the accumulation of lipid in L-02 cells, improved alcoholic liver injury in rats, alleviated hepatic pathological lesions, and reduced lipid deposition in rat serum and liver. Further studies suggest that MFA reduces lipid synthesis by activating AMPK-ACC/MAPK-FoxO1 pathway. In addition, MFA also promotes lipid oxidation by up-regulating the expression of SIRT1, PPAR-α, and CPT-1α. Taken together, MFA ameliorates ethanol-induced hepatic steatosis by activating AMPK-ACC/MAPK-FoxO1 pathway and up-regulating the expression levels of SIRT1, PPAR-α, and CPT-1α.
[Lignan glycosides and sucrose esters from roots of Securidaca inappendiculata].[Pubmed: 28822186]
Nine compounds, including five lignan glycosides (1-5), three sucrose esters (6-8), and one organic acid ester (9), were isolated from the ethanol extract of the roots of Securidaca inappendiculata by various chromatographic methods including silica gel, MPLC and preparative HPLC. Their structures were elucidated as acernikol-4″-O-β-D-glucopyranoside (1), (7R, 8S)-dihydrodehydrodiconiferyl alcohol 9-O-β-D-glucopyranoside (2), (7R, 8S)-dihydrodehydrodiconiferyl alcohol 4-O-β-D-glucopyranoside (3), (7R, 8S)-dihydrodehydrodiconiferyl alcohol 9'-O-β-D-glucopyranoside (4), (7R, 8S)-5-methoxydihydrodehy-drodiconiferyl alcohol 4-O-β-D-glucopyranoside (5), 3, 6'-O-diferuloylsucrose (6), 3-O-feruloyl-6'-O-sinapoylsucrose (7), sibricose A5 (8), and mehyl ferulate (9) on the basis of 1H-, 13C-NMR and MS experiments. Compounds 1-5, 8, and 9 were isolated from the Securidaca genus for the first time. Compounds 2, 3, and 7 exhibited weak cytotoxic activities against Hela and MCF-7 cell lines.
Reactive oxygen species mediated NF-κB/p38 feedback loop implicated in proliferation inhibition of HFLS-RA cells induced by 1,7-dihydroxy-3,4-dimethoxyxanthone.[Pubmed: 28810523]
1,7-Dihydroxy-3,4-dimethoxyxanthone (XAN) is a bioactive compound isolated from Securidaca inappendiculata Hassk. and exerts the inhibitory effects on fibroblast-like synoviocytes by targeting NF-κB and p38. This study was designed to elucidate mechanisms underlying the divergent regulation on the two pathways in HFLS-RA cells by XAN. Expressions of hallmark proteins and transcription of GADD45α mRNA were determined by Western-blot and RT-qPCR methods, respectively. Fluorescence staining was employed to evaluate intracellular oxidative stress. Effects of XAN and N-acetyl-l-cysteine (NAC) on the proliferation of cells were investigated by MTT assay, and pro-apoptotic effects of XAN were assessed by Annexin V-FITC/PI method. It was found XAN blocked NF-κB signaling in HFLS-RA cells shortly after treatment. Moreover, it up-regulated both transcription and expression of GADD45α, and subsequently activated p38 pathway. As time went on, XAN significantly promoted the generation of reactive oxygen species (ROS), which accompanied with sustained up-regulation of p-p38 and increased apoptosis. 48H later, dual-effects of XAN on NF-κB and p38 were reversed. As activation of p38 and increased apoptosis induced by XAN were antagonized by NAC, they were deemed as ROS mediated effects. Furthermore, the accumulated ROS should also account for the activation of NF-κB in the late stage of treatments via interfering in p38/MSK1/NF-κB feedback. Altogether, these findings suggested XAN-induced ROS contributed great importance to the proliferation inhibition of HFLS-RA cells by mediating NF-κB/p38 feedback loop and apoptosis, which provided us a panoramic view of potential target in the therapy of RA by XAN.
Structure-activity relationships of diverse xanthones against multidrug resistant human tumor cells.[Pubmed: 28065566]
Thirteen xanthones were isolated naturally from the stem of Securidaca inappendiculata Hassk, and structure-activity relationships (SARs) of these compounds were comparatively predicted for their cytotoxic activity against three human multidrug resistant (MDR) cell lines MCF-7/ADR, SMMC-7721/Taxol, and A549/Taxol cells. The results showed that the selected xanthones exhibited different potent cytotoxic activity against the growth of different human tumor cell lines, and most of the xanthones exhibited selective cytotoxicity against SMMC-7721/Taxol cells. Furthermore, some tested xanthones showed stronger cytotoxicity than Cisplatin, which has been used in clinical application extensively. The SARs analysis revealed that the cytotoxic activities of diverse xanthones were affected mostly by the number and position of methoxyl and hydroxyl groups. Xanthones with more free hydroxyl and methoxyl groups increased the cytotoxic activity significantly, especially for those with the presence of C-3 hydroxyl and C-4 methoxyl groups.
Regulation of MAPKs Signaling Contributes to the Growth Inhibition of 1,7-Dihydroxy-3,4-dimethoxyxanthone on Multidrug Resistance A549/Taxol Cells.[Pubmed: 27403196]
1,7-Dihydroxy-3,4-dimethoxyxanthone (XAN) is a bioactive compound isolated from Securidaca inappendiculata Hassk. and validated with antiproliferative activities on a panel of cancer cell lines. This study was designed to investigate its growth inhibitory effects on multidrug resistance (MDR) non-small cell lung carcinoma (NSCLC) cell line A549/Taxol and explore the possible linkage between modulation of MAPKs and the bioactivities. Its growth inhibitory potency on the cells was estimated by MTT assay, and flow cytometric analysis was employed to investigate its potential cell cycle arrest and proapoptosis effects. Expressions of hallmark proteins were assessed by Western-Blot method. The results showed A549/Taxol cells were sensitive to XAN. XAN inhibited the proliferation of A549/Taxol cells in the time and concentration dependent manners. It acted as a potent inducer of apoptosis and cell cycle arrest in the cells. Western-Blot investigation validated the proapoptosis and cell cycle arrest activities of XAN and the potential of MDR reversion. Upregulation of p38 by XAN, which accounted for the cell cycle arrest at G2 phase, and the downregulation of ERK associated with the proapoptosis activity were also revealed. Further analysis found p53 may be the central role mediated the bioactivities of MAPKs in A549/Taxol cells. Based on these evidences, a conclusion has been deduced that XAN could be a potential agent for MDR NSCLC therapy targeting specifically MAPKs.
[Saponins from roots of Securidaca inappendiculata with cytotoxic activities].[Pubmed: 26666038]
Seven acylated triterpene saponins were isolated from the roots of Securidaca inappendiculata by means of various chromatographic techniques such as silica gel, MPLC, preparative HPLC, and semi-preparative HPLC. Their chemical structures were identified as securioside A(1), securioside B(2), 3-O-β-D-glucopyranosyl presenegenin 28-O-β-D-xylopyranosyl-(1-->4)-α-L-rhamnopyranosyl-(1-->2)-[β-D-glucopyranosyl-(1-->3)]-4-O-[(E)-3,4-dimethoxycinnamoyl]-β-D-fucopyranosyl ester(3), 3-O-β-D-glucopyranosyl presenegenin 28-O-β-D-xylopyranosyl-(1-->4)-α-L-rhamnopyranosyl-(1-->2)-[β-D-glucopyranosyl-(1-->3) ] -4-O-[(E/Z)-3, 4-dimethoxycinnamoyl]-β-D-fucopyranosyl ester(3/4), 3-O-β-D-glucopyranosyl presenegenin 28-O-α-L-arabinopyranosyl-(1-->3)-β-D-xylopyranosyl-(1-->4)-α-L-rhamnopyranosyl-(1-->2)-4-O-[(E)-3,4-dimethoxycinnamoyl]-β-D-fucopyranosyl ester(5), polygalasa- ponin XLV(6), and polygalasaponin XLVI (7) on the basis of spectroscopic data analysis and physicochemical properties. Among them, compounds 5-7 were isolated from the plants in genus Securidaca for the first time and compounds 3, 3/4 were isolated from the species for the first time. The cytotoxicity assay showed that compounds 2, 3/4, 5 have moderate cytotoxic activities against Lewis lung carcinoma LLC cells with IC50 values of 41.10, 38.17, and 48.92 µmol · L(-1), respectively; compound 2 exhibited moderate cytotoxic activities against human breast cancer MCF-7 cells with an IC50 value of 47.93 µmol · L(-1).
Selective modulation of MAPKs contribute to the anti-proliferative and anti-inflammatory activities of 1,7-dihydroxy-3,4-dimethoxyxanthone in rheumatoid arthritis-derived fibroblast-like synoviocyte MH7A cells.[Pubmed: 25862966]
1,7-Dihydroxy-3,4-dimethoxyxanthone (XAN) is an antirheumatic agent isolated from traditional Chinese medicine Securidaca inappendiculata Hassk. This study was designed to investigate its anti-proliferative and anti-inflammatory activities on rheumatoid arthritis derived fibroblast-like synoviocyte cell line MH7A, and explore the underlying mechanism of action.
Xanthone-rich dichloromethane fraction of Securidaca inappendiculata, the possible antirheumatic material base with anti-inflammatory, analgesic, and immunodepressive effects.[Pubmed: 25026334]
Securidaca inappendiculata Hassk. is an traditional Chinese medicine curing rheumatoid arthritis, but there is a lack of reports on material base research.
Xanthones as α-glucosidase inhibitors from the antihyperglycemic extract of Securidaca inappendiculata.[Pubmed: 24621306]
Securidaca inappendiculata Hassk. (SI) is used to cure fractures and rheumatoid arthritis in China. Also, it is a potential antidiabetes drug; however, there are no reports on this.


