Selaginella pulvinata
Selaginella pulvinata
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Selaginella pulvinata
- Cat.No. Product Name CAS Number COA
-
BCN6283
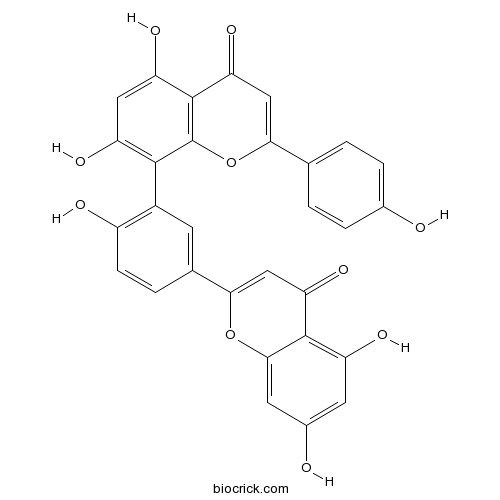
Amentoflavone1617-53-4
Instructions
Diselaginellin B, an Unusual Dimeric Molecule from Selaginella pulvinata, Inhibited Metastasis and Induced Apoptosis of SMMC-7721 Human Hepatocellular Carcinoma Cells.[Pubmed: 29144743]
Two new unusual dimeric selaginellins, diselaginellins A and B (1 and 2), along with two known derivatives, selaginellin (3) and selaginellin B (4), were isolated from Selaginella pulvinata. Their structures were elucidated by extensive NMR and high-resolution ESIMS data analysis. Compound 2 displayed apoptosis-inducing and antimetastatic activities against the human hepatocellular carcinoma cell line SMMC-7721. A microarray analysis revealed that genes related to metabolism, angiogenesis, and metastasis were altered by 2. The up- and down-regulation of the mRNA levels of related genes was confirmed by RT-qPCR. Metabolism modulation and metastasis inhibition might be the mechanisms of the antitumor properties of diselaginellin B (2).
A new selaginellin derivative and a new triarylbenzophenone analog from the whole plant of Selaginella pulvinata.[Pubmed: 28952358]
None
The discovery, complex crystal structure, and recognition mechanism of a novel natural PDE4 inhibitor from Selaginella pulvinata.[Pubmed: 28159622]
None
A new selaginellin derivative from Selaginella pulvinata.[Pubmed: 25975028]
A new selaginellin derivative named as selaginellin S (1) was isolated from the whole plants of Selaginella pulvinata (Hook. et Grev.) Maxim. (Selaginellaceae), together with a known one (selaginellin M, 2). Compounds 1 and 2 were separated and purified by silica gel and Sephadex LH-20 column chromatography. Their structures were determined on the basis of extensive spectroscopic analysis including IR, MS, 1D and 2D NMR experiments, as well as ECD calculations. Compound 1 is a key intermidiant in the biosynthesis pathway of selaginellins. Compound 2 is first reported in this plant.
Selaginpulvilins A-D, new phosphodiesterase-4 inhibitors with an unprecedented skeleton from Selaginella pulvinata.[Pubmed: 24328835]
Selaginpulvilins A-D (1-4), four new phenols with an unprecedented 9,9-diphenyl-1-(phenylethynyl)-9H-fluorene skeleton, together with four known selaginellins (5-8) were isolated from Selaginella pulvinata. Their structures were elucidated by spectroscopic analysis and chemical correlation. The structure of 1 was confirmed by single-crystal X-ray diffraction. Compounds 1-8 exhibited remarkable inhibitory activities (IC50 values in the range of 0.11-5.13 μM) against phosphodiesterase-4 (PDE4), a drug target for the treatment of asthma and chronic obstructive pulmonary disease.
Cloning and truncation modification of trehalose-6-phosphate synthase gene from Selaginella pulvinata.[Pubmed: 23069851]
A homologous sequence was amplified from resurrection plant Selaginella pulvinta by RACE technique, proved to be the full-length cDNA of trehalose-6-phosphate synthase gene by homologous alignment and yeast complementation assay, and nominated as SpTPS1 gene. The open reading frame of this gene was truncated 225bp at the 5'-end, resulting the N-terminal truncation modification of 75 amino acids for its encoding protein. The TPS1 deletion mutant strain YSH290 of the brewer's yeast transformed by the truncated gene SpTPS1Δ and its original full-length version restored growth on the medium with glucose as a sole carbon source and displayed growth curves with no significant difference, indicating their encoding proteins functioning as TPS enzyme. The TPS activity of the mutant strain transformed by the truncated gene SpTPS1Δ was about six fold higher than that transformed by its original version, reasoning that the extra N-terminal extension of the full-length amino acid sequence acts as an inhibitory domain to trehalose synthesis. However, the trehalose accumulation of the mutant strain transformed by the truncated gene SpTPS1Δ was only 8% higher than that transformed by its original version. This result is explained by the feedback balance of trehalose content coordinated by the comparative activities between trehalose synthase and trehalase. The truncated gene SpTPS1Δ is suggested to be used in transgenic operation, together with the inhibition of trehalase activity by the application of validamycin A or genetic deficiency of the endogenous trehalase gene, for the enhancement of trehalose accumulation and improvement of abiotic tolerance in transgenic plants.
Selaginellin M, a new selaginellin derivative from Selaginella pulvinata.[Pubmed: 21985670]
A new selaginellin derivative, selaginellin M (1), together with one known compound, selaginellin E (2), was isolated from Selaginella pulvinata. The structure of the new compound was elucidated and named as (R,S)-4-((4'-hydroxy-4-((2-hydroxyethoxy)methyl))-3-((4-hydroxyphenyl)ethynyl)biphenyl-2-yl)(4-hydroxyphenyl)methylene)cyclohexa-2,5-dienone on the basis of the spectroscopic data including UV, IR, 1D, and 2D NMR as well as HR-ESI-MS analysis.
Structure determination of selaginellins G and H from Selaginella pulvinata by NMR spectroscopy.[Pubmed: 20623720]
Selaginellins G (1) and H (2), two new selaginellin derivatives, were isolated from the whole plant of Selaginella pulvinata. Their structures were elucidated, and complete assignments of the (1)H and (13)C NMR spectroscopic data were achieved by 1D and 2D NMR experiments (HSQC, HMBC, COSY and ROESY). Compound 1 displayed good antifungal activity against Candida albicans with an IC(50) value of 5.3 microg/ml.


