Solanum torvum
Solanum torvum
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Solanum torvum
- Cat.No. Product Name CAS Number COA
-
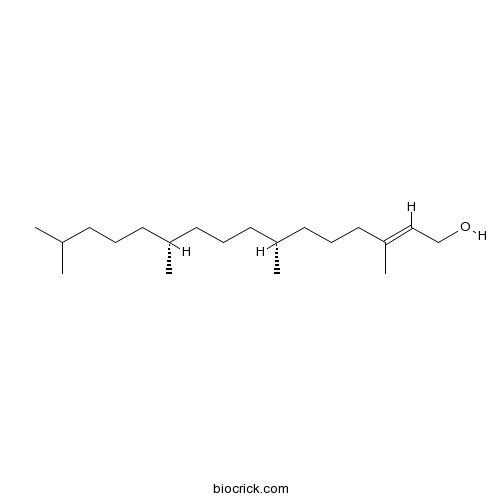
BCN1673
Phytol150-86-7
Instructions
-
BCN4546
4-Hydroxybenzoic acid99-96-7
Instructions

A Solanum torvum GH3 β-glucosidase expressed in Pichia pastoris catalyzes the hydrolysis of furostanol glycoside.[Pubmed: 27055587]
Plant β-glucosidases are usually members of the glucosyl hydrolase 1 (GH1) or 3 (GH3) families. Previously, a β-glucosidase (torvosidase) was purified from Solanum torvum leaves that specifically catalyzed hydrolysis of two furostanol 26-O-β-glucosides, torvosides A and H. Furostanol glycoside 26-O-β-glucosides have been reported as natural substrates of some plant GH1 enzymes. However, torvosidase was classified as a GH3 β-glucosidase, but could not hydrolyze β-oligoglucosides, the natural substrates of GH3 enzymes. Here, the full-length cDNA encoding S. torvum β-glucosidase (SBgl3) was isolated by the rapid amplification of cDNA ends method. The 1887bp ORF encoded 629 amino acids and showed high homology to other plant GH3 β-glucosidases. Internal peptide sequences of purified native Sbgl3 determined by LC-MS/MS matched the deduced amino acid sequence of the Sbgl3 cDNA, suggesting that it encoded the natural enzyme. Recombinant SBgl3 with a polyhistidine tag (SBgl3His) was successfully expressed in Pichia pastoris. The purified SBgl3His showed the same substrate specificity as natural SBgl3, hydrolyzing torvoside A with much higher catalytic efficiency than other substrates. It also had similar biochemical properties and kinetic parameters to the natural enzyme, with slight differences, possibly attributable to post-translational glycosylation. Quantitative real-time PCR (qRT-PCR) showed that SBgl3 was highly expressed in leaves and germinated seeds, suggesting a role in leaf and seedling development. To our knowledge, a recombinant GH3 β-glucosidase that hydrolyzes furostanol 26-O-β-glucosides, has not been previously reported in contrast to substrates of GH1 enzymes.
Broad taxonomic characterization of Verticillium wilt resistance genes reveals an ancient origin of the tomato Ve1 immune receptor.[Pubmed: 26946045]
Plant-pathogenic microbes secrete effector molecules to establish themselves on their hosts, whereas plants use immune receptors to try and intercept such effectors in order to prevent pathogen colonization. The tomato cell surface-localized receptor Ve1 confers race-specific resistance against race 1 strains of the soil-borne vascular wilt fungus Verticillium dahliae which secrete the Ave1 effector. Here, we describe the cloning and characterization of Ve1 homologues from tobacco (Nicotiana glutinosa), potato (Solanum tuberosum), wild eggplant (Solanum torvum) and hop (Humulus lupulus), and demonstrate that particular Ve1 homologues govern resistance against V. dahliae race 1 strains through the recognition of the Ave1 effector. Phylogenetic analysis shows that Ve1 homologues are widely distributed in land plants. Thus, our study suggests an ancient origin of the Ve1 immune receptor in the plant kingdom.
Nine sesquiterpenes from Solanum torvum.[Pubmed: 26824767]
Three new sesquiterpenes, namely 3β,11-dihydroxy-4,14-oxideenantioeudesmane (1), 1β,10β,12,14-tetrahydroxy-allo-aromadendrane (2) and 1β,10β,13,14-tetrahydroxy-allo-aromadendrane (3), along with six known sesquiterpenes (4-9), were isolated from the roots of Solanum torvum. Compound 4 and 5 are epimers, their main difference lies in the C-11 configulation. Normally, epimers do not make a huge difference in C NMR spectra, but in this kind of structure of A, B, C rings, and C ring is sterically strained structure, stericall effects influence strongly the (13)C NMR chemical shifts, when C-11 configulation changed, it makes a huge difference in the three ring of structure, such as C-6, C-7, C-11. New compound 2 and 3 are epimers and similar to compound 4 and 5, their just increase a hydroxy in C-1 and have a same regular pattern in C NMR spectra, otherwise, compound 5 was firstly confirmed by single-crystal X-ray diffraction.
Solanum torvum Swartz. fruit attenuates cadmium-induced liver and kidney damage through modulation of oxidative stress and glycosylation.[Pubmed: 26762936]
Increased levels of environmental pollutants are linked to almost all human disorders; the efficient method to manage the human health is through naturally available dietary molecule. Solanum torvum (ST) Swartz (Solanaceae) commonly called Turkey Berry is found in Africa, Asia, and South America. Its fruit, part of traditional Indian cuisine, is a widely consumed nutritious herb, acclaimed for its medicinal value. ST aqueous extract (STAe) (250, 500, and 1000 mg/kg b.w., 6 days; oral) against acute Cadmium (Cd) (6.3 mg/kg b.w., single dose; oral) toxicity was evaluated in rats. Protective effect was assessed using serum markers, tissue antioxidants, oxidant derivatives, glycoprotein, and histopathological studies. The activities of serum marker enzymes were increased (40-60 %); antioxidant enzymes such as SOD and CAT, GSH, and its metabolic enzyme activities were decreased (50-80 %) in the liver and kidney upon Cd intoxication. During STAe pre-treatment, at doses of 250 and 500 mg/kg b.w., the above changes were brought to near normal (25-63 %). Tissue 4-hydroxynonenal, 3-nitrotyrosine, and protein carbonyls were increased (8-15 fold) in Cd-alone-treated rats, whereas pre-supplementation of STAe significantly decreased their levels and inhibited the protein glycosylation effectively. The pharmacological effect of STAe was confirmed by histopathological observations. Based on previous literature and present investigation, we conclude that ST may serve as a potential functional food against environmental contaminant such as heavy metal-induced oxidative stress.


