Stellaria yunnanensis
Stellaria yunnanensis
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Stellaria yunnanensis
- Cat.No. Product Name CAS Number COA
-
BCN2916
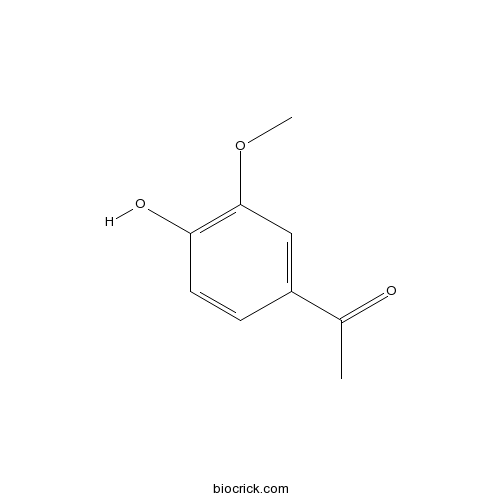
Acetovanillone498-02-2
Instructions
Cyclization of several linear penta- and heptapeptides with different metal ions studied by CD spectroscopy.[Pubmed: 15686535]
A cyclic pentapeptide c(Tyr-Leu-Ala-Gly-Pro) (I), which was isolated and identified from Pseudostellaria heterophylla medicinal herbs, and two cyclic heptapeptides, c(Gly-Tyr-Gly-Gly-Pro-Phe-Pro) (II) and c(Gly-Ile-Pro-Tyr-Ile-Ala-Ala) (III), which were isolated and identified from Stellaria yunnanensis Franch (M), were synthesized by using 3-(diethoxyphosphoryloxy)-1,2,3-benzotriazin-4(3 H)-one (DEPBT) as a coupling reagent in solution, and mediated by different metal ions, from their linear peptide precursors H-Tyr-Leu-Ala-Gly-Pro-OH (I-1) and H-Ala-Gly-Pro-Tyr-Leu-OH (I-2), H-Gly-Tyr-Gly-Gly-Pro-Phe-Pro-OH (II-1) and H-Gly-Ile-Pro-Tyr-Ile-Ala-Ala-OH (III-1), respectively. The results show that alkali metal ions can improve the cyclization yields and/or the cyclization rates of linear peptide precursors, such as Na(+) ion is favorable for the cyclization of linear pentapeptides and Cs(+) ion is favorable for the cyclization of linear heptapeptides, while some bivalent and trivalent metal ions, such as Mg(2+), Ca(2+), Zn(2+), Fe(2+), Ni(2+) and Cr(3+) reduced/inhibited both the cyclization yields and the cyclization rates of the linear peptide precursors. The circular dichroism spectra of I-1, II-1 and III-1 with different metal ions were studied to elucidate the changes in their secondary structures. It is shown that Cs(+) can induce and stabilize the type I beta-turn conformation in the linear heptapeptide II-1 and the type II beta-turn conformation in the linear heptapeptide III-1.
Cyclic peptides from higher plants. 24.1 yunnanin C, a novel cyclic heptapeptide from Stellaria yunnanensis.[Pubmed: 8882429]
A novel cytotoxic cyclic heptapeptide, yunnanin C (1), was isolated from the roots of Stellaria yunnanensis. The structure of 1, cyclo(-Gly-Ile-Gly-Phe-Tyr-Ser-Pro-), was elucidated from spectroscopic evidence and by chemical degradation.
Cyclopeptides from Stellaria yunnanensis.[Pubmed: 8534403]
In a previous paper, we reported the structural elucidation of stellarin A, a new cyclic heptapeptide, from the fresh roots of Stellarina yunnanensis Franch. Further chemical study on this plant led to the isolation of another two new cyclopeptides named stellarin B and C. Their structures were established to be cyclo(Gly-Ser-HOIle-Phe-Phe-Ala) and cyclo(Gly-Ser-HOIle-Phe-Phe-Ser), respectively, by spectral methods.


