Stephania epigaea
Stephania epigaea
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Stephania epigaea
- Cat.No. Product Name CAS Number COA
-
BCN5393
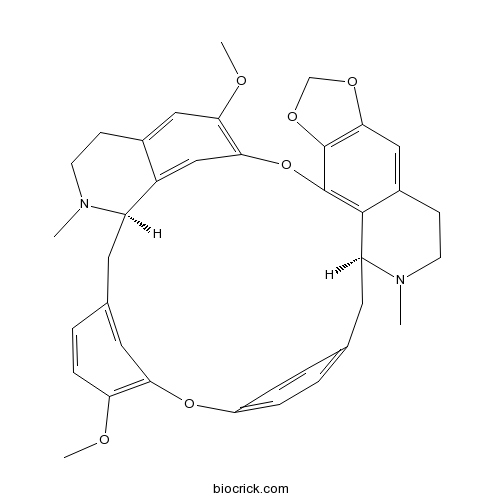
Cepharanthine481-49-2
Instructions
-
BCN8445
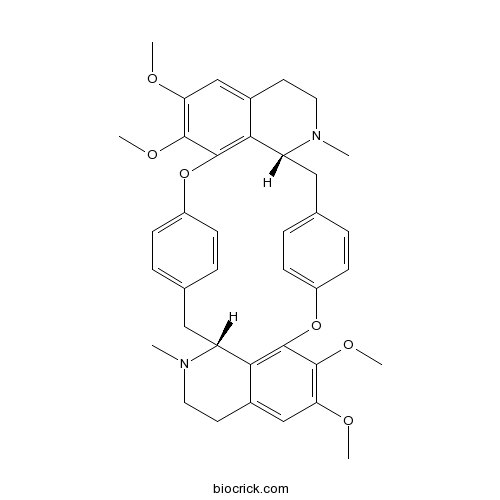
Cycleanine518-94-5
Instructions
Proaporphine and aporphine alkaloids with acetylcholinesterase inhibitory activity from Stephania epigaea.[Pubmed: 26028544]
An unusual proaporphine alkaloid bearing an isopropanenitrile group at isoquinoline nitrogen, named epiganine A (1) and a new aporphine alkaloid, epiganine B (2), together with eight known alkaloids, pronuciferine (3), dehydrodicentrine (4), romerine (5), romeline (6), N-methylcalycinine (7), phanostenine (8), dicentrine (9), and N-methyllaurotetanine (10), were isolated from the roots of Stephania epigaea. The absolute configuration of 1 was determined by calculating electronic circular dichroism (ECD) and comparing with experimental data. Compounds 2 and 4 showed strong acetylcholinesterase (AChE) inhibitory effects with the IC50 values of 4.36 and 2.98μM, respectively. Compounds 5-9 also exhibited potent AChE inhibitory activities.
Cytotoxic bisbenzylisoquinoline alkaloids from Stephania epigaea.[Pubmed: 23621840]
Six new bisbenzylisoquinoline alkaloids (1-6) and seven known compounds (8-14) were isolated from the tubers of Stephania epigaea, in addition to the major alkaloid, cepharanthine (7). The structures of 1-6 were elucidated by combined spectroscopic data analysis and chemical methods, with their configurations determined from their optical rotation values and confirmed using circular dichroism. Compounds 1-6 belong to the oxyacanthine type of bisbenzylisoquinoline alkaloids and have a rare methylenedioxy substituent. Compound 1, a dimer composed of benzylisoquinoline and seco-aristolactam units, represents a new type of bisbenzylisoquinoline alkaloid, while compounds 3-6 are bisbenzylisoquinoline N-oxides. These compounds were evaluated for their in vitro cytotoxicities against six human cancer cell lines (A-549, ECA109, HL-60, MCF-7, SMMC-7721, and SW480). Cepharanthine (7), the major component of S. epigaea, exhibited cytotoxicity against all of these cancer cell lines except ECA109, while its known analogue, 10, displayed cytotoxicity against all six cancer cell lines.


