Streptocaulon juventas
Streptocaulon juventas
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Streptocaulon juventas
- Cat.No. Product Name CAS Number COA
-
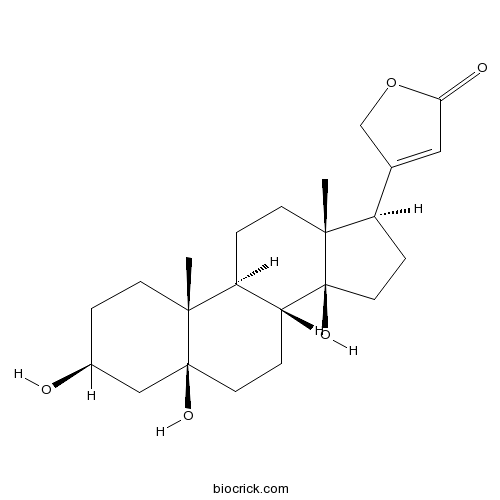
BCN2656
Periplogenin514-39-6
Instructions
Wound healing activity of Streptocaulon juventas root ethanolic extract.[Pubmed: 29219215]
Streptocaulon juventas is a well-known plant that has antimicrobial activity, in vitro antiplasmodial activity, anti-proliferative activity, and antioxidant activity. In this study, we showed experimental evidence that proved that S. juventas root ethanolic extract has wound healing activities. First, in a mouse excision wound model, S. juventas root ethanolic extract at a dose of 100 mg/kg/day significantly reduced the wound closure time. After 7 days, the wound granulation tissue in mice treated with the extract exhibited a 2.3-fold decrease in inflammatory cells, a 1.7-fold increase in fibroblasts and enhanced angiogenesis. Molecular analysis also revealed that after wounds were treated with S. juventas root ethanolic extract, TNF-α and NF-κB1 gene expression were down-regulated by 4.7 and 3.7 times, respectively. In contrast, TGF-β1 and VEGF gene expression were up-regulated by 1.9 and 6.5 times, respectively. Taken together, our experimental data strongly show that the ethanolic extract from S. juventas root displays remarkable wound healing activity.
TXA9, a cardiac glycoside from Streptocaulon juventas, exerts a potent anti-tumor activity against human non-small cell lung cancer cells in vitro and in vivo.[Pubmed: 25555472]
Non-small cell lung cancer is the most common type of lung cancer and the most common cause of cancer-related death in humans. TXA9, which is a natural product separated from an anti-tumor-active fraction of the roots of Streptocaulon juventas, may possess potent anti-proliferative activity according to the present study. In this study, the anti-tumor effects and toxicity of TXA9 were tested against human non-small cell lung cancer cell lines (A549, NCI-H1299, Ltep-α2, PC-9, and Lu99) and a normal human lung embryonic fibroblast cell (HE-lung) in vitro, and then toward A549 cells in vivo in a murine xenograft model. The results show that TXA9 exhibits potent cytotoxic activities against non-small lung cancer cells and has no toxic effect on the normal human lung embryonic fibroblast cells. The mechanistic studies demonstrate that TXA9 can induce the apoptosis of A549 cells through the extrinsic pathway. The in vivo study results reveal that the intravenous administration of TXA9 at high-dose (15 mg kg(-1)) induces significant tumor growth inhibition of non-small cell lung cancer xenografts with tumor inhibition rate up to 64.2%, compared with mice in the control group. The inhibitory effect was similar to that of taxol (62.5%). In particular, no significantly adverse effects were exerted by TXA9, which suggests that it is well tolerated. This promising natural product may be useful as a potential novel anti-tumor candidate.
Minor cytotoxic cardenolide glycosides from the root of Streptocaulon juventas.[Pubmed: 25449765]
In order to determine new minor natural cardenolide glycosides as cytotoxic candidates, we isolated six new cardenolide glycosides together with four known ones, which had never previously been reported in the genus, by bioassay-guided separation from the 75% ethanol extract of Streptocaulon juventas (Asclepiadaceae). Their structures were elucidated on the basis of spectroscopic analysis, including homo- and heteronuclear correlation NMR experiments (COSY, HSQC and HMBC). The cytotoxic activities of these compounds were evaluated against A549 and NCI-H460 cell lines by MTT assay and compound 7 exhibited inhibitory activity against the two cell lines, while other compounds displayed a range of inhibitory activity against NCI-H460 and A549 cells. Their structure-activity relationships were also discussed.
The cytotoxic activities of cardiac glycosides from Streptocaulon juventas and the structure-activity relationships.[Pubmed: 25128424]
A series of cardiac glycosides were isolated and identified from the anti-tumor fraction of the root of Streptocaulon juventas in previous studies. In the present research, the cytotoxic activities of the 43 cardiac glycosides on three cell lines, human lung A549 adenocarcinoma cell, large cell lung cancer NCI-H460 cell and normal human fetal lung fibroblast MRC-5 cell, were evaluated in vitro. Most of the tested compounds showed potent inhibitory activities toward the three cell lines. Then, the structure-activity relationships were discussed in detail. It was indicated that hydroxyl and acetyl groups at C-16 increased the activity, whereas hydroxyl group at C-1 and C-5 can both increase and decrease the activity. Two glucosyl groups which were connected by C1'→C6' showed better inhibitory activity against cancer cell lines, while the C1'→C4' connection showed stronger inhibitory activity against the normal cell line. Also, this is the first report that the activities of these compounds exhibited different variation trends between A549 and NCI-H460 cell lines, which indicated that these compounds could selectively inhibit the cell growth. The results would lay a foundation for further research on new anti-tumor drug development.
Cardenolide glycosides from root of Streptocaulon juventas.[Pubmed: 23286880]
Six cardenolides were isolated from the anti-tumor active fraction of the 75% ethanol extract of Streptocaulon juventas (Asclepiadaceae), mainly found in southwest of China. These were named 1α, 14β-dihydroxy-5β-card-20 (22)-enolide 3-O-[O-β-D-glucopyranosyl-(1→2)-β-D-digitalopyranoside] (1), acovenosigenin A 3-O-[O-β-D-glucopyranosyl-(1→4)-β-D-digitalopyranoside] (2), 16-O-acetyl-hydroxyperiplogenin 3-O-β-D-digitoxopyranoside (3), digitoxigenin 3-O-[O-β-D-glucopyranosyl-(1→6)-O-β-D-glucopyranosyl-(1→4)-2-O-acetyl-β-D-digitalopyranoside] (4), 16-O-acetyl-hydroxyacovenosigenin (5), 1β, 3β, 14β-trihydroxy-5β-card-16, 20 (22)-dienolide (6), together with 18 known ones, most of which had never been reported in the species beforehand. Their structures were elucidated on the basis of spectroscopic studies and comparison with reference. Eighteen compounds were evaluated for their cytotoxicity against human lung A549 adenocarcinoma cell lines.
Cytotoxic cardiac glycosides from the roots of Streptocaulon juventas.[Pubmed: 23225367]
Chemical investigation on the 75% ethanol extract of the roots of Streptocaulon juventas afforded two new cardiac glycosides, digitoxigenin 3-O-[O-β-D-glucopyranosyl-(1 → 4)-2-O-acetyl-β-D-digitalopyranoside] (1) and periplogenin 3-O-[O-β-D-glucopyranosyl-(1 → 4)-O-β-D-glucopyranosyl-(1 → 4)-β-D-cymaropyranoside] (2), and thirteen known cardenolides. Structures were elucidated by spectral methods. This is the first report of the isolation of compounds 3, 10, 14, and 15 from plants of the Streptocaulon genus, while 4, 11, and 12 are hitherto unreported from Streptocaulon juventas. All the compounds were in vitro evaluated for their cytotoxic activities against the A549 cell line, and seven effective cardiac glycosides were screened against the PC-9 cell line by WST assay, which also showed strong antiproliferation activities. Moreover, the characteristic morphological changes in PC-9 cells treated with cardenolides indicated cell inhibition due to apoptosis. These results revealed that these compounds possessed potential antitumor activities.
Inhibitory activity of a phytochemically characterized fraction from Streptocaulon juventas on lung cancer in nude mice.[Pubmed: 19918714]
In the present study, a 75% ethanol extract of Streptocaulon juventas (SJ), which had a strong inhibitory effect on the proliferation of human lung A549 adenocarcinoma cells, was subjected to bioassay-guided fractionation. The most active fraction (SJF) was obtained using a macroreticular resin column followed by a silica-gel column. Then its in vivo effect on lung cancer was investigated in athymic nude mice with A549 tumors while its effects on body weight, blood biochemical indicators, and organ indices were monitored. The results showed that SJF inhibited the tumor growth significantly at day 10 and day 15 during treatment without physical side effects. Following HPLC and NMR spectrometry, the main components of SJF were identified as digitoxigenin, periplogenin, and periplogenin glucoside.
Constituents of the Vietnamese medicinal plant Streptocaulon juventas and their antiproliferative activity against the human HT-1080 fibrosarcoma cell line.[Pubmed: 14640513]
The methanolic extract of roots of Streptocaulon juventas, having shown strong antiproliferative activity against the highly metastatic human HT-1080 fibrosarcoma cell line, was subjected to activity-guided isolation to yield 16 cardenolides including five new ones, acovenosigenin A 3-O-beta-digitoxopyranoside (1), digitoxigenin gentiobioside (2), digitoxigenin 3-O-[O-beta-glucopyranosyl-(1-->6)-O-beta-glucopyranosyl-(1-->4)-3-O-acetyl-beta-digitoxopyranoside] (3), digitoxigenin 3-O-[O-beta-glucopyranosyl-(1-->6)-O-beta-glucopyranosyl-(1-->4)-O-beta-digitalopyranosyl-(1-->4)-beta-cymaropyranoside] (4), and periplogenin 3-O-(4-O-beta-glucopyranosyl-beta-digitalopyranoside) (5), and two new hemiterpenoids, (4R)-4-hydroxy-3-isopropylpentyl beta-rutinoside (6) and (R)-2-ethyl-3-methylbutyl beta-rutinoside (7), together with two known phenylpropanoids and a known phenylethanoid. The isolated cardenolides strongly inhibited the proliferation of the HT-1080 cell line (IC(50) values, 54-1600 nM).
Antiproliferative activity of cardenolides isolated from Streptocaulon juventas.[Pubmed: 14519950]
Sixteen cardenolides, two hemiterpenoids, two phenylpropanoids and a phenylethanoid isolated from the roots of Streptocaulon juventas (LOUR.) MERR. were examined for their antiproliferative activity toward three human-derived (HT-1080 fibrosarcoma, lung A549 adenocarcinoma, cervix HeLa adenocarcinoma) and three murine-derived (colon 26-L5 carcinoma, Lewis lung carcinoma, B16-BL6 melanoma) cell lines. The cardenolides selectively and strongly inhibited proliferation of the HT-1080 (IC(50) values, 0.054-1.6 microM) and A549 (IC(50), 0.016-0.65 microM) cell lines. The characteristic morphological changes and ladder-like DNA fragmentation in those cells treated with the cardenolides indicated the antiproliferative activity was due to the induction of apoptosis.
Antiproliferative activity of Vietnamese medicinal plants.[Pubmed: 12081142]
Methanol, methanol-water (1:1) and water extracts were prepared from seventy-seven Vietnamese medicinal plants and tested for their antiproliferative activities against human HT-1080 fibrosarcoma cells. Among them, fifteen extracts including seven methanol extracts of Caesalpinia sappan, Catharanthus roseus, Coscinium fenestratum, Eurycoma longifolia, Hydnophytum formicarum and Streptocaulon juventas (collected at two areas), six methanol-water (1:1) extracts of Cae. sappan, Cat. roseus, Co. fenestratum, H. formicarum and S. juventas (at two areas), and two water extracts of Cae. sappan and S. juventas exhibited antiproliferative activities in a concentration-dependent manner. Their antiproliferative activities against human cervix HeLa adenocarcinoma, human lung A549 adenocarcinoma, murine colon 26-L5 carcinoma, murine Lewis lung carcinoma (LLC) and murine B16-BL6 melanoma cells were then examined. Co. fenestratum showed selective activity against lung carcinoma and/or lung metastatic cell lines, A549, LLC and B16-BL6, while H. formicarum and S. juventas showed selective activity against human tumor cell lines, HeLa and A549. Characteristic morphological change and DNA fragmentation indicated the antiproliferative activity to be due to the induction of apoptosis.


