Strobilanthes cusia
Strobilanthes cusia
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.

Natural products/compounds from Strobilanthes cusia
- Cat.No. Product Name CAS Number COA
-
BCN5528
Betulin473-98-3
Instructions

-
BCN2385
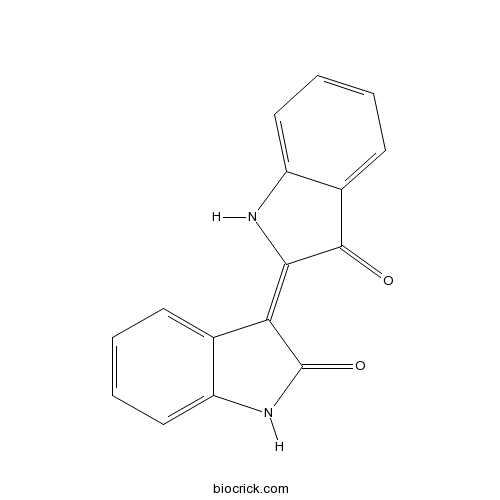
Indirubin479-41-4
Instructions
-
BCC2448
4-HQN491-36-1
Instructions

Indirubin, a component of Ban-Lan-Gen, activates CYP3A4 gene transcription through the human pregnane X receptor.[Pubmed: 26987505]
Ban-Lan-Gen is the common name for the dried roots of indigo plants, including Polygonum tinctorium, Isatis indigotica, Isatis tinctoria, and Strobilanthes cusia. Ban-Lan-Gen is frequently used as an anti-inflammatory and an anti-viral for the treatment of hepatitis, influenza, and various types of inflammation. One of the cytochrome P450 (CYP) enzymes, CYP3A4, is responsible for the metabolism of a wide variety of xenobiotics, including an estimated 60% of all clinically used drugs. In this study, we investigated the effect of Ban-Lan-Gen on the transcriptional activation of the CYP3A4 gene. Ban-Lan-Gen extract increased CYP3A4 gene reporter activity in a dose-dependent manner. Indirubin, one of the biologically active ingredients in the Ban-Lan-Gen, also dose-dependently increased CYP3A4 gene reporter activity. Expression of short hairpin RNA for the human pregnane X receptor (hPXR-shRNA) inhibited CYP3A4 gene reporter activity, and overexpression of human PXR increased indirubin- and rifampicin-induced CYP3A4 gene reporter activity. Furthermore, indirubin induced CYP3A4 mRNA expression in HepG2 cells. Taken together, these results indicate that indirubin, a component of Ban-Lan-Gen, activated CYP3A4 gene transcription through the activation of the human PXR.
A novel isocoumarin with anti-influenza virus activity from Strobilanthes cusia.[Pubmed: 26506123]
Strobilanthes A (1), a novel isocoumarin with an unusual tetrahydro-4H-pyran-4-one moiety fused isocoumarin core skeleton, together with a known compound (2) was isolated from Strobilanthes cusia. Its chemical structures were elucidated by 2D NMR spectroscopy, mass spectrometry and single-crystal X-ray diffraction analysis. The biosynthetic pathway of 1 could be supposed to be originally derived from 3-methylisocoumarin, a product of AA-MA pathway. Both of two compounds displayed anti-influenza virus activity in vitro.
Rapid Identification and Verification of Indirubin-Containing Medicinal Plants.[Pubmed: 26089942]
Indirubin, one of the key components of medicinal plants including Isatis tinctoria, Polygonum tinctorium, and Strobilanthes cusia, possesses great medicinal efficacy in the treatment of chronic myelocytic leukemia (CML). Due to misidentification and similar name, materials containing indirubin and their close relatives frequently fall prey to adulteration. In this study, we selected an internal transcribed spacer 2 (ITS2) for distinguishing these indirubin-containing species from five of their usual adulterants, after assessing identification efficiency of matK, rbcL, psbA-trnH, and ITS2 among these species. The results of genetic distances and neighbor-joining (NJ) phylogenetic tree indicated that ITS2 region is a powerful DNA barcode to accurately identify these indirubin-containing species and discriminate them from their adulterants. Additionally, high performance liquid chromatography (HPLC) was used to verify indirubin in different organs of the above species. The results showed that indirubin had been detected in the leaves of Is. tinctoria, P. tinctorium, S. cusia, and Indigo Naturalis (made from their mixture), but not in their roots, or in the leaves of their adulterants. Therefore, this study provides a novel and rapid method to identify and verify indirubin-containing medicinal plants for effective natural treatment of CML.
Indole alkaloid glycosides from the aerial parts of Strobilanthes cusia.[Pubmed: 25427242]
Three indole alkaloid glycosides, strobilanthosides A-C (1-3), two known indole alkaloid glucosides (4 and 5), and five phenylethanoid glycosides (8-10) were isolated from the aerial parts of Strobilanthes cusia. The structures of the new compounds were elucidated by spectrometric analysis, and the absolute configurations of 1 and 2 were established by ECD spectrocsopy. N'-β-d-Glucopyranosylindirubin (5) showed weak antibacterial activity (MIC 62.5-125 μM) against Staphylococcus aureus.
New bactericide derived from Isatin for treating oilfield reinjection water.[Pubmed: 22929650]
Isatin, an extract from Strobilanthes cusia (Nees) Kuntze, was the base for synthesizing derivatives that were screened for antibacterial activity against oilfield water-borne bacteria. The bacterial groups are sulfate reducing, iron and total. The derivatives were characterized by spectrums and they showed good to moderate activity against sulfate reducing bacteria.
3-Acetonyl-3-hydroxyoxindole: a new inducer of systemic acquired resistance in plants.[Pubmed: 18266823]
Systemic acquired resistance (SAR) is an inducible defence mechanism which plays a central role in protecting plants from microbial pathogen attack. Guided by bioassays, a new chemical inducer of SAR was isolated from the extracts of Strobilanthes cusia and identified to be 3-acetonyl-3-hydroxyoxindole (AHO), a derivative of isatin. Tobacco plants treated with AHO exhibited enhanced resistance to tobacco mosaic virus (TMV) and to the fungal pathogen Erysiphe cichoracearum (powdery mildew), accompanied by increased levels of pathogenesis-related gene 1 (PR-1) expression, salicylic acid (SA) accumulation and phenylalanine ammonia-lyase activity. To study the mode of action of AHO, its ability to induce PR-1 expression and TMV resistance in nahG transgenic plants expressing salicylate hydroxylase, which prevents the accumulation of SA, was analysed. AHO treatment did not induce TMV resistance or PR-1 expression in nahG transgenic plants, suggesting that AHO acts upstream of SA in the SAR signalling pathway. In addition, using two-dimensional gel electrophoresis combined with mass spectrometry, five AHO-induced plant proteins were identified which were homologous to the effector proteins with which SA interacts. Our data suggest that AHO may represent a novel class of inducer that stimulates SA-mediated defence responses.
Seco-pregnane steroids target the subgenomic RNA of alphavirus-like RNA viruses.[Pubmed: 17470783]
Plants have evolved multiple mechanisms to selectively suppress pathogens by production of secondary metabolites with antimicrobial activities. Therefore, direct selections for antiviral compounds from plants can be used to identify new agents with potent antiviral activity but not toxic to hosts. Here, we provide evidence that a class of compounds, seco-pregnane steroid glaucogenin C and its monosugar-glycoside cynatratoside A of Strobilanthes cusia and three new pantasugar-glycosides of glaucogenin C of Cynanchum paniculatum, are effective and selective inhibitors to alphavirus-like positive-strand RNA viruses including plant-infecting tobacco mosaic virus (TMV) and animal-infecting Sindbis virus (SINV), eastern equine encephalitis virus, and Getah virus, but not to other RNA or DNA viruses, yet they were not toxic to host cells. In vivo administration of the compounds protected BALB/c mice from lethal SINV infection without adverse effects on the mice. Using TMV and SINV as models, studies on the action mechanism revealed that the compounds predominantly suppress the expression of viral subgenomic RNA(s) without affecting the accumulation of viral genomic RNA. Our work suggested that the viral subgenomic RNA could be a new target for the discovery of antiviral drugs, and that seco-pregnane steroid and its four glycosides found in the two medicinal herbs have the potential for further development as antiviral agents against alphavirus-like positive-strand RNA viruses.


