Taxus cuspidata
Taxus cuspidata
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Taxus cuspidata
- Cat.No. Product Name CAS Number COA
-
BCN2514
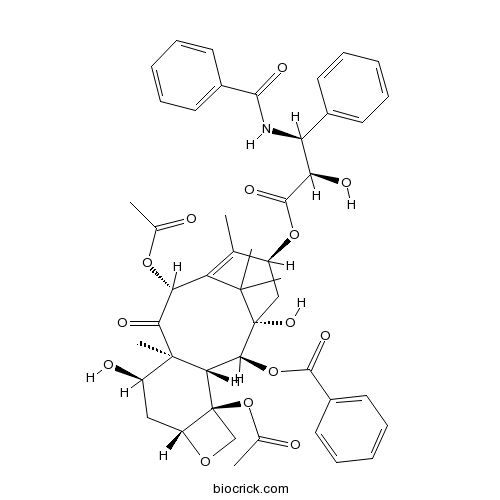
7-Epitaxol105454-04-4
Instructions
-
BCN8306
Methyl Oleate112-62-9
Instructions

-
BCN5343
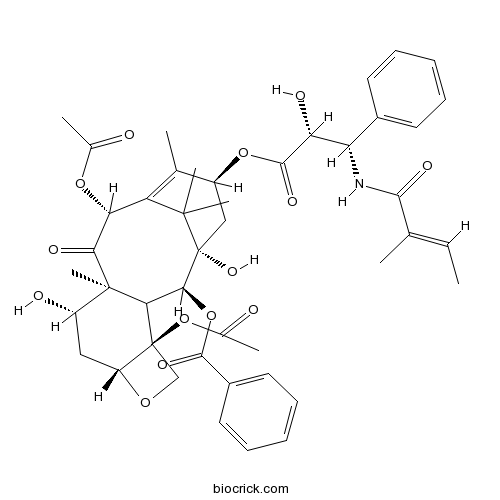
Cephalomannine71610-00-9
Instructions
Taxadiene-5α-ol is a minor product of CYP725A4 when expressed in Escherichia coli.[Pubmed: 28876471]
CYP725A4 is a P450 enzyme from Taxus cuspidata that catalyzes the formation of taxadiene-5α-ol (T5α-ol) from taxadiene in paclitaxel biosynthesis. Past attempts expressing CYP725A4 in heterologous hosts reported the formation of 5(12)-oxa-3(11)-cyclotaxane (OCT) and/or 5(11)-oxa-3(11)-cyclotaxane (iso-OCT) instead of, or in addition to, T5α-ol. Here, we report that T5α-ol is produced as a minor product by Escherichia coli expressing both taxadiene synthase and CYP725A4. The major products were OCT and iso-OCT, while trace amounts of unidentified monooxygenated taxanes were also detected by gas chromatography-mass spectrometry. Since OCT and iso-OCT had not been found in nature, we tested the hypothesis that protein-protein interaction of CYP725A4 with redox partners, such as cytochrome P450 reductase (CPR) and cytochrome b5, may affect the products formed by CYP725A4, possibly favoring the formation of T5α-ol over OCT and iso-OCT. Our results show that coexpression of CYP725A4 with CPR from different organisms did not change the relative ratios of OCT, iso-OCT, and T5α-ol, while cytochrome b5 decreased overall levels of the products formed. Although unsuccessful in finding conditions that promote T5α-ol formation over other products, we used our results to clarify conflicting claims in the literature and discuss other possible approaches to produce paclitaxel via metabolic and enzyme engineering.
Development of polymorphic microsatellite markers for Japanese yew, Taxus cuspidata, and T. cuspidata var. nana (Taxaceae).[Pubmed: 27437172]
Taxus cuspidata (Taxaceae), which is well known for the effective anticancer metabolite paclitaxel (e.g., taxol), is an evergreen needle-leaved tree widely distributed in eastern Eurasia including Japan. We developed 15 microsatellite markers from this species and confirmed their utility for the dwarf variety nana, which is common in alpine regions along the Sea of Japan.
Immunostimulating activity of polyhydric alcohol isolated from Taxus cuspidata.[Pubmed: 26791584]
A polyhydric alcohol (PAL) was isolated from Taxus cuspidata and its immunostimulatory activities were assessed. The primary monosaccharide composition of the PAL was determined to be glucose, where HPAEC analysis showed no significant amount of any other sugars. However, glycerol and xylitol were identified as the main sugar alcohols. Fourier-transform infrared (FT-IR) analysis indicated that the purified PAL is a complex glycitol, which structurally contains significant amount of hydroxyl groups. MALDI-TOF mass spectroscopy also demonstrated that PAL is a complex glycitol built in hexose polymerization. Enzyme linked immunosorbent assay showed that the PAL stimulates the release of the proinflammatory cytokines TNF-α and IL-6 in a dose-dependent manner. Furthermore, treatment of RAW 264.7 cells with PAL for 24h remarkably increased the phosphorylation levels of ERK, p38 and JNK in a dose-dependent manner, whereas the total protein levels of ERK (t-ERK), p38 (t-p38) and JNK (t-JNK) remained unchanged. These results clearly demonstrate that PAL stimulates the immune response in RAW 264.7 cells through the activation of MAPKs (ERK, p38 and JNK) signaling pathway. To the best of our knowledge, this is the first study to demonstrate the primary structure and immune-stimulating activities of PAL from the fruit of T. cuspidata.
Mechanistic Insights into Taxadiene Epoxidation by Taxadiene-5α-Hydroxylase.[Pubmed: 26677870]
The anticancer molecule taxol (Paclitaxel) stands as one of the most medically and economically important natural products. However, despite decades of extensive study, its biosynthesis remains poorly understood. Unpredictable behavior of the first oxygenation enzyme, taxadiene-5α-hydroxylase, which produces a range of undesired products, currently stands as a key bottleneck to improved taxol production. We herein present chemical and biological evidence of an unreported epoxidase activity of taxadiene-5α-hydroxylase that puts into question the previously proposed radical-rebound mechanism. We demonstrate that the poor selectivity of taxadiene-5α-hydroxylase arises from nonselective degradation of an epoxide intermediate produced via a selective oxidation step, rather than from promiscuous oxidation, as previously proposed. We support these conclusions by demonstrating variable enzyme behavior in differing hosts and conditions, similarity of products and product ratios generated from chemical epoxidation, and taxadiene-5α-hydroxylase, and differing enzymatic activity on alternative taxadiene isomers. Additionally, we use directed mutagenesis to describe the oxidizing species of the P450, demonstrate that further in vivo functionalization of oxidized taxadiene is unable to improve selectivity of the oxidation, and show that multiple products are produced in the Taxus cuspidata and are not simply an artifact of heterologous expression. Our results highlight an important, and previously unknown, obstacle to improved taxol production. We further offer insights to overcome the challenges posed by an epoxide-mediated reaction, which sets the basis for further engineering of taxol biosynthesis.
A process optimization study on ultrasonic extraction of paclitaxel from Taxus cuspidata.[Pubmed: 25830908]
This study aimed to improve the extraction rate of paclitaxel from Taxus cuspidata in order to determine the most effective combination of ultrasonic extraction and thin-layer chromatography-ultraviolet (TLC-UV) rapid separation method. The study was performed using the Box-Behnken test design to conduct single-factor experiments using ultrasonic extraction of paclitaxel from Taxus cuspidata. The study showed ethanol to be the best extraction solvent. When mixed with dichloromethane (1:1), the ratio of material to liquid was 1:50 when using an ultrasonic time of 1 hr at a power of 200 W. The correction coefficient K for the separation and detection of paclitaxel using the TLC-UV spectrophotometric method was 0.009152. Multifactor experiments determined the effect of the rate of liquid to material (X1), ultrasonic time (X2), and ultrasonic power (X3) on extraction using extraction volume as the dependent variable. Response surface analysis allowed a regression equation to be obtained, with the optimal conditions for extraction when the rate of liquid to material was 53.23 mL/g as an ultrasonic time of 1.11 hr and an ultrasonic power of 207.88 W. Using these parameters, the average amount of extracted paclitaxel was about 130.576 µg/g, which was significantly better than for other extraction methods.
Jasmonate-responsive expression of paclitaxel biosynthesis genes in Taxus cuspidata cultured cells is negatively regulated by the bHLH transcription factors TcJAMYC1, TcJAMYC2, and TcJAMYC4.[Pubmed: 25767476]
Taxus cell suspension culture is a sustainable technology for the industrial production of paclitaxel (Taxol®), a highly modified diterpene anti-cancer agent. The methyl jasmonate (MJ)-mediated paclitaxel biosynthetic pathway is not fully characterized, making metabolic engineering efforts difficult. Here, promoters of seven genes (TASY, T5αH, DBAT, DBBT, PAM, BAPT, and DBTNBT), encoding enzymes of the paclitaxel biosynthetic pathway were isolated and used to drive MJ-inducible expression of a GUS reporter construct in transiently transformed Taxus cells, showing that elicitation of paclitaxel production by MJ is regulated at least in part at the level of transcription. The paclitaxel biosynthetic pathway promoters contained a large number of E-box sites (CANNTG), similar to the binding sites for the key MJ-inducible transcription factor AtMYC2 from Arabidopsis thaliana. Three MJ-inducible MYC transcription factors similar to AtMYC2 (TcJAMYC1, TcJAMYC2, and TcJAMYC4) were identified in Taxus. Transcriptional regulation of paclitaxel biosynthetic pathway promoters by transient over expression of TcJAMYC transcription factors indicated a negative rather than positive regulatory role of TcJAMYCs on paclitaxel biosynthetic gene expression.
Expression of recombinant alpha and beta tubulins from the yew Taxus cuspidata and analysis of the microtubule assembly in the presence of taxol.[Pubmed: 25070196]
Taxol was originally isolated from the yew Taxus brevifolia. Because taxol inhibits the depolymerization of microtubules, the presence of a self-resistance mechanism in Taxus spp. was hypothesized. The cloning of the cDNA for alpha and beta tubulins from Taxus cuspidata and those from the human embryonic kidney cell line HEK293T revealed that the (26)Asp, (359)Arg, and (361)Leu residues in the human beta tubulin, which are important for taxol binding, were replaced with Glu, Trp, and Met in the beta tubulin of T. cuspidata, respectively. The microtubule assembly of the recombinant alpha and beta tubulins was monitored turbidimetrically, and the results clearly demonstrated that the microtubule from T. cuspidata is less sensitive to taxol than that from HEK293T cells. The Taxus microtubule composed of the wild-type alpha tubulin and the beta tubulin with the E26D mutation restored the sensitivity to taxol. We thus postulated that the mutation identified in the beta tubulin of T. cuspidata plays a role in the self-resistance of this species against taxol.
Methyl jasmonate represses growth and affects cell cycle progression in cultured Taxus cells.[Pubmed: 24832773]
Methyl jasmonate elicitation of Taxus cultures enhances paclitaxel accumulation, but represses growth by inhibition of cell cycle progression. Growth repression is evident both at the culture level and transcriptional level. Methyl jasmonate (MeJA) elicitation is an effective strategy to induce and enhance synthesis of the anticancer agent paclitaxel (Taxol(®)) in Taxus cell suspension cultures; however, concurrent decreases in growth are often observed, which is problematic for large-scale bioprocessing. Here, increased accumulation of paclitaxel in Taxus cuspidata suspension cultures with MeJA elicitation was accompanied by a concomitant decrease in cell growth, evident within the first 3 days post-elicitation. Both MeJA-elicited and mock-elicited cultures exhibited similar viability with no apoptosis up to day 16 and day 24 of the cell culture period, respectively, suggesting that growth repression is not attributable to cell death. Flow cytometric analyses demonstrated that MeJA perturbed cell cycle progression of asynchronously dividing Taxus cells. MeJA slowed down cell cycle progression, impaired the G1/S transition as observed by an increase in G0/G1 phase cells, and decreased the number of actively dividing cells. Through a combination of deep sequencing and gene expression analyses, the expression status of Taxus cell cycle-associated genes correlated with observations at the culture level. Results from this study provide valuable insight into the mechanisms governing MeJA perception and subsequent events leading to repression of Taxus cell growth.


