Uncaria macrophylla
Uncaria macrophylla
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Uncaria macrophylla
- Cat.No. Product Name CAS Number COA
-
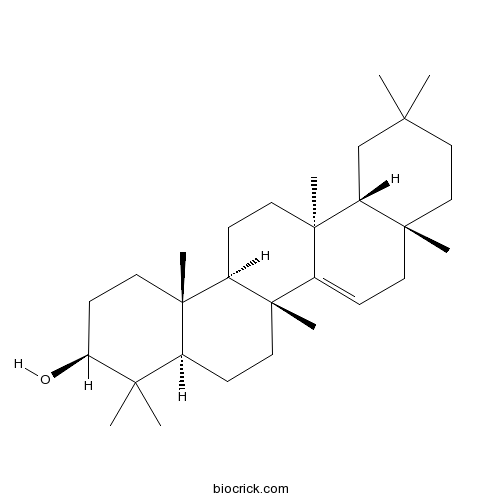
BCN6148
Taraxerol127-22-0
Instructions
-
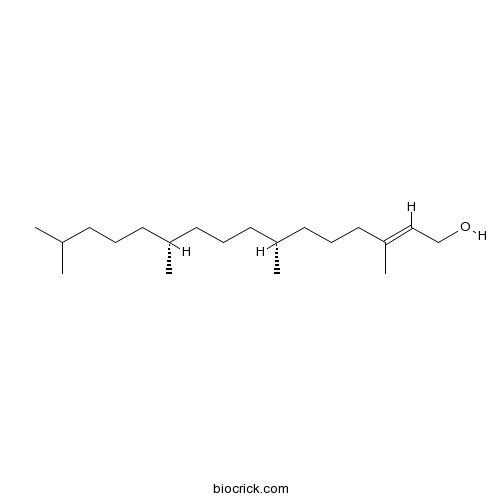
BCN1673
Phytol150-86-7
Instructions
-
BCN8454
Corynoxine B17391-18-3
Instructions

-
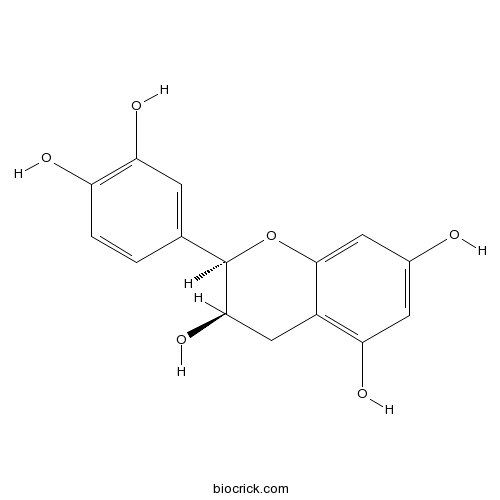
BCN5597
Epicatechin490-46-0
Instructions
-
BCN2364
Corynoxine6877-32-3
Instructions

-
BCN4979
Rhynchophylline76-66-4
Instructions

-
BCN4327
Ursolic acid77-52-1
Instructions

The effects of genetic variation and environmental factors on rhynchophylline and isorhynchophylline in Uncaria macrophylla Wall. from different populations in China.[Pubmed: 29953548]
Uncaria macrophylla Wall. is an important Chinese medicinal herb. Rhynchophylline (RIN) and isorhynchophylline (IRN) are its major active compounds. We investigated the influence of genetic differentiation and environmental factors on the RIN and IRN to find the main influencing factors of their contents and lay the foundation for the following cultivation and breeding. We used inter-simple sequence repeat (ISSR) markers to investigate the genetic diversity, and high-performance liquid chromatography (HPLC) to measure the contents of RIN and IRN in 200 samples of U. macrophylla obtained from nine natural populations, and then to analyze the correlation between genetic differentiation, environmental factors of sampling sites and the contents of RIN and IRN. We found that High intra-population (80.05%) and low inter-population (19.95%) genetic diversity existed in the samples of U. macrophylla. To some extent, genetic differentiation and the contents of RIN and IRN had correlation in individual populations (such as JH, MH, XM, and ML). The RIN and IRN contents were significant negatively correlated with the precipitation in May (RIRN = -0.771, p = 0.015) and June (RRIN = -0.814, p = 0.008; RIRN = -0.921, p = 0.000), indicating that precipitation was the main affecting factor of their contents. Interestingly, the analysis results showed that the RIN content had a significant positive correlation (r = 0.585, p = 0.000) with the IRN content (they are isomers); the proportion of RIN had a significant negative correlation with the sum of the two (r = -0.390, p<0.0001), while the proportion of IRN had a significant positive correlation (r = 0.390, p<0.0001). It meant that, with the total quantity of the two compounds increased, the proportion of RIN decreased and the proportion of IRN increased, illustrating that their conversion exist some regularity. Moreover, the content ratio of RIN and IRN was significant positively correlated with the January precipitation (r = 0.716, p = 0.030), implying that January may be the key period for the mutual transformation of RIN and IRN.
Supercritical fluid chromatography for separation and preparation of tautomeric 7-epimeric spiro oxindole alkaloids from Uncaria macrophylla.[Pubmed: 27843099]
None
Preparative separation of six rhynchophylla alkaloids from Uncaria macrophylla wall by pH-zone refining counter-current chromatography.[Pubmed: 24352009]
pH-Zone refining counter-current chromatography was successfully applied to the preparative isolation and purification of six alkaloids from the ethanol extracts of Uncaria macrophylla Wall. Because of the low content of alkaloids (about 0.2%, w/w) in U. macrophylla Wall, the target compounds were enriched by pH-zone refining counter-current chromatography using a two-phase solvent system composed of petroleum ether-ethyl acetate-isopropanol-water (2:6:3:9, v/v), adding 10 mM triethylamine in organic stationary phase and 5 mM hydrochloric acid in aqueous mobile phase. Then pH-zone refining counter-current chromatography using the other two-phase solvent system was used for final purification. Six target compounds were finally isolated and purified by following two-phase solvent system composed of methyl tert-butyl ether (MTBE)-acetonitrile-water (4:0.5:5, v/v), adding triethylamine (TEA) (10 mM) to the organic phase and HCl (5 mM) to aqueous mobile phase. The separation of 2.8 g enriched total alkaloids yielded 36 mg hirsutine, 48 mg hirsuteine, 82 mg uncarine C, 73 mg uncarine E, 163 mg rhynchophylline, and 149 mg corynoxeine, all with purities above 96% as verified by HPLC Their structures were identified by electrospray ionization-mass spectrometry (ESI-MS) and 1H-NMR spectroscopy.
A new triterpene From Uncaria macrophylla and its antitumor activity.[Pubmed: 22334066]
On our ongoing investigation, a new oleanolic triterpene, 3β,6β,19α-trihydroxy-12-oleanen-28-oic acid (1) was obtained from the chloroform-soluble portion of the 90% alcohol-water extract of the stem bark of Uncaria macrophylla. Its structure was elucidated by extensive spectroscopic methods, including 1D and 2D ((1)H-(1)H COSY, HSQC and HMBC) NMR and HR-ESI-MS. The cytotoxicities of the compound was evaluated against two cancer cell lines of MCF-7 and HepG2 by the MTT method, and the compound exhibited weak activities with the IC(50) values of 78.2 µg/mL and 73.9 µg/mL.
A new triterpene from the plant of uncaria macrophylla.[Pubmed: 22222909]
Our ongoing investigations on the stem bark of Uncaria macrophylla afforded a new ursolic triterpene, 3β,6β,19α-trihydroxy-urs-12-en-28-oic acid-24-carboxylic acid methyl ester (1), named uncariursanic acid, and three known ursolic triterpenes including 3β,6β,19α-trihydroxy-23-oxo-urs-12-en-28-oic acid (2), 3β,6β,19α-trihydroxy-urs-12-en-28-oic acid (3) and ursolic acid (4). Their structures were elucidated by extensive spectral methods, including 1D and 2D NMR and HR-ESI-MS. The cytotoxicities of the four compounds were evaluated against two cancer cell lines (MCF-7 and HepG2) by the MTT method, and only compound 4 exhibited potent activity.
Macrophyllionium and macrophyllines A and B, oxindole alkaloids from Uncaria macrophylla.[Pubmed: 21070010]
An unusual oxindole alkaloid inner salt, macrophyllionium (1), and a pair of new tetracyclic oxindole alkaloids, macrophyllines A (2) and B (3), together with six known alkaloids, were isolated from the aerial parts of Uncaria macrophylla. Corynantheidine (8) exhibited moderate cytotoxicity against HL-60 and SW480 cells with IC(50) values of 13.96 and 23.28 μM, respectively. Dihydrocorynantheine (9) exhibited significant vasodilating activity against phenylephrine-induced contraction in rat thoracic aorta rings (IC(50) = 6.73 μg/mL). In addition, compounds 2, 6, and 9 showed weak inhibitory action on KCl-induced contraction.
[Chemical constituents of the non-alkaloid fraction of Uncaria macrophylla].[Pubmed: 17355944]
To analyze the chemical constituents of the non-alkaloid fraction of Uncaria macrophylla.


