cis-JasmoneCAS# 488-10-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 488-10-8 | SDF | Download SDF |
| PubChem ID | 1549018 | Appearance | Oil |
| Formula | C11H16O | M.Wt | 164.2 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
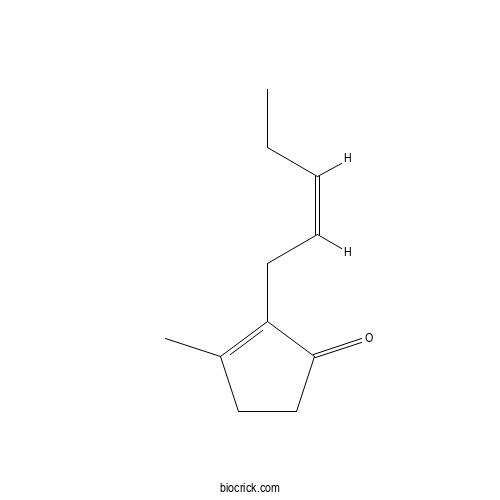
| Chemical Name | 3-methyl-2-[(Z)-pent-2-enyl]cyclopent-2-en-1-one | ||
| SMILES | CCC=CCC1=C(CCC1=O)C | ||
| Standard InChIKey | XMLSXPIVAXONDL-PLNGDYQASA-N | ||
| Standard InChI | InChI=1S/C11H16O/c1-3-4-5-6-10-9(2)7-8-11(10)12/h4-5H,3,6-8H2,1-2H3/b5-4- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | cis-Jasmone controls indirect plant defence through a distinct signalling pathway. It induces Arabidopsis genes that affect the chemical ecology of multitrophic interactions with aphids and their parasitoids. | |||||

cis-Jasmone Dilution Calculator

cis-Jasmone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.0901 mL | 30.4507 mL | 60.9013 mL | 121.8027 mL | 152.2533 mL |
| 5 mM | 1.218 mL | 6.0901 mL | 12.1803 mL | 24.3605 mL | 30.4507 mL |
| 10 mM | 0.609 mL | 3.0451 mL | 6.0901 mL | 12.1803 mL | 15.2253 mL |
| 50 mM | 0.1218 mL | 0.609 mL | 1.218 mL | 2.4361 mL | 3.0451 mL |
| 100 mM | 0.0609 mL | 0.3045 mL | 0.609 mL | 1.218 mL | 1.5225 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Dihydroisoferulic acid
Catalog No.:BCN9898
CAS No.:1135-15-5
- 3',4'-Dimethoxyflavone
Catalog No.:BCN9897
CAS No.:4143-62-8
- Decanoic acid
Catalog No.:BCN9896
CAS No.:334-48-5
- 3-Octanone
Catalog No.:BCN9895
CAS No.:106-68-3
- Methyl anthranilate
Catalog No.:BCN9894
CAS No.:134-20-3
- (-)-Linalool
Catalog No.:BCN9893
CAS No.:126-91-0
- 5-Geranoxy-7-methoxycoumarin
Catalog No.:BCN9892
CAS No.:7380-39-4
- Caffeoyl alcohol
Catalog No.:BCN9891
CAS No.:3598-26-3
- Tryptamine hydrochloride
Catalog No.:BCN9890
CAS No.:343-94-2
- Cinnamtannin A2
Catalog No.:BCN9889
CAS No.:86631-38-1
- Tribulosin
Catalog No.:BCN9888
CAS No.:79974-46-2
- 4-Oxadocosane-1,2-diol
Catalog No.:BCN9887
CAS No.:544-62-7
- Methyl benzoate
Catalog No.:BCN9900
CAS No.:93-58-3
- Bufotenine
Catalog No.:BCN9901
CAS No.:487-93-4
- 3-Methyl ellagic acid
Catalog No.:BCN9902
CAS No.:51768-38-8
- 5-Methyl-3-heptanone
Catalog No.:BCN9903
CAS No.:541-85-5
- 3-O-Acetyl 9,11-dehydro beta-boswellic acid
Catalog No.:BCN9904
CAS No.:122651-20-1
- Eupalitin
Catalog No.:BCN9905
CAS No.:29536-41-2
- Eupalitin 3-galactoside
Catalog No.:BCN9906
CAS No.:35399-32-7
- Calycopterin
Catalog No.:BCN9907
CAS No.:481-52-7
- Epoxybergamottin
Catalog No.:BCN9729
CAS No.:206978-14-5
- Corynanthine
Catalog No.:BCN9910
CAS No.:483-10-3
- 5-Methoxypiperonal
Catalog No.:BCN9911
CAS No.:5780-07-4
- (R)-O-isobutyroyllomatin
Catalog No.:BCN9912
CAS No.:440094-38-2
Double Gamers-Can Modified Natural Regulators of Higher Plants Act as Antagonists against Phytopathogens? The Case of Jasmonic Acid Derivatives.[Pubmed:33213072]
Int J Mol Sci. 2020 Nov 17;21(22). pii: ijms21228681.
As key players in biotic stress response of plants, jasmonic acid (JA) and its derivatives cover a specific and prominent role in pathogens-mediated signaling and hence are promising candidates for a sustainable management of phytopathogenic fungi. Recently, JA directed antimicrobial effects on plant pathogens has been suggested, supporting the theory of oxylipins as double gamers in plant-pathogen interaction. Based on these premises, six derivatives (dihydrojasmone and cis-Jasmone, two thiosemicarbazonic derivatives and their corresponding complexes with copper) have been evaluated against 13 fungal species affecting various economically important herbaceous and woody crops, such as cereals, grapes and horticultural crops: Phaeoacremonium minimum, Neofusicoccum parvum, Phaeomoniella chlamydospora, Fomitiporia mediterranea, Fusarium poae, F. culmorum, F. graminearum, F. oxysporum f. sp. lactucae,F. sporotrichioides, Aspergillus flavus, Rhizoctonia solani,Sclerotinia spp. and Verticillium dahliae. The biological activity of these compounds was assessed in terms of growth inhibition and, for the two mycotoxigenic species A. flavus and F. sporotrichioides, also in terms of toxin containment. As expected, the inhibitory effect of molecules greatly varied amongst both genera and species; cis-Jasmone thiosemicarbazone in particular has shown the wider range of effectiveness. However, our results show that thiosemicarbazones derivatives are more effective than the parent ketones in limiting fungal growth and mycotoxins production, supporting possible applications for the control of pathogenic fungi.
Jasmonates: biosynthesis, perception and signal transduction.[Pubmed:32602544]
Essays Biochem. 2020 Sep 23;64(3):501-512.
Jasmonates (JAs) are physiologically important molecules involved in a wide range of plant responses from growth, flowering, senescence to defence against abiotic and biotic stress. They are rapidly synthesised from alpha-linolenic acid (ALA; C18:3 9,12,15) by a process of oxidation, cyclisation and acyl chain shortening involving co-operation between the chloroplast and peroxisome. The active form of JA is the isoleucine conjugate, JA-isoleucine (JA-Ile), which is synthesised in the cytoplasm. Other active metabolites of JA include the airborne signalling molecules, methyl JA (Me-JA) and cis-Jasmone (CJ), which act as inter-plant signalling molecules activating defensive genes encoding proteins and secondary compounds such as anthocyanins and alkaloids. One of the key defensive metabolites in many plants is a protease inhibitor that inactivates the protein digestive capabilities of insects, thereby, reducing their growth. The receptor for JA-Ile is a ubiquitin ligase termed as SCFCoi1 that targets the repressor protein JA Zim domain (JAZ) for degradation in the 26S proteasome. Removal of JAZ allows other transcription factors (TFs) to activate the JA response. The levels of JA-Ile are controlled through catabolism by hydroxylating enzymes of the cytochrome P450 (CYP) family. The JAZ proteins act as metabolic hubs and play key roles in cross-talk with other phytohormone signalling pathways in co-ordinating genome-wide responses. Specific subsets of JAZ proteins are involved in regulating different response outcomes such as growth inhibition versus biotic stress responses. Understanding the molecular circuits that control plant responses to pests and pathogens is a necessary pre-requisite to engineering plants with enhanced resilience to biotic challenges for improved agricultural yields.
Jasmonic acid is not a biosynthetic intermediate to produce the pyrethrolone moiety in pyrethrin II.[Pubmed:32286354]
Sci Rep. 2020 Apr 14;10(1):6366.
Pyrethrum (Tanacetum cinerariifolium) produces insecticidal compounds known as pyrethrins. Pyrethrins are esters; the acid moiety is either trans-chrysanthemic acid or pyrethric acid and the alcohol moiety of pyrethrins is either pyrethrolone, cinerolone, or jasmolone. It was generally accepted that cis-Jasmone was biosynthetic intermediate to produce the alcohol moieties of pyrethrin, and the biosynthetic origin of the cis-Jasmone was postulated to be jasmonic acid. However, there was no direct evidence to prove this hypothesis. In order to uncover the origin of pyrethrolone moiety in pyrethrin II, feeding experiments were performed employing deuterium- and (13)C-labeled compounds as substrates, and the expected labeled compounds were analyzed using UPLC MS/MS system. It was found that the pyrethrolone moiety in pyrethrin II was derived from 12-oxo-phytodienoic acid (OPDA), iso-OPDA and cis-Jasmone but not from methyl jasmonate and 3-oxo-2-(2'-[Z]-pentenyl)-cyclopentane-1-hexanoic acid. The results supported that the biosynthesis of the pyrethrolone moiety in pyrethrin II partially used part of the jasmonic acid biosynthetic pathway, but not whole.
Association between olfactory sensitivity and behavioral responses of Drosophila suzukii to naturally occurring volatile compounds.[Pubmed:32190926]
Arch Insect Biochem Physiol. 2020 Jul;104(3):e21669.
Drosophila suzukii Matsumura (Diptera: Drosophilidae) is an invasive, destructive crop pest that originated in South East Asia. D. suzukii recently invaded Western countries and is threatening both European and American fruit industries. It is extremely attracted to otherwise undamaged, ripening fruits, unlike most other Drosophila species that attack only decaying or rotten fruits. Recent studies on different insect species showed that several naturally occurring compounds of easy market availability showing deterrent action may be used to supplement mass catches with food traps. Based on these considerations, the aim of the present work was to test the effects of some natural compounds (alone or in the mixture) on the olfactory system of the D. suzukii and the behavioral responses evoked. We measured by electroantennogram (EAG) recordings, the olfactory sensitivity of antennae to increasing concentrations of eugenol, vanillin, menthol, cis-Jasmone; eugenol + vanillin, +menthol, +cis-Jasmone; vanillin + menthol, +cis-Jasmone. In addition, the behavioral responses to the same compounds and mixtures were evaluated. Our electrophysiological results show a dose-response relationship between the EAG amplitudes and the increasing concentrations of the olfactory compound. The behavioral results show that the number of laid eggs is significantly different between the standard diet and the standard diet + natural compound. These results underline a specificity in the olfactory sensitivity and in the ovipositing behavior of D. suzukii females; also, they could be valuable for the identification of key chemicals aimed at the future development of strategies in the management and control of this harmful insect for crops.
Transcriptional Variation in Glucosinolate Biosynthetic Genes and Inducible Responses to Aphid Herbivory on Field-Grown Arabidopsis thaliana.[Pubmed:31572432]
Front Genet. 2019 Sep 11;10:787.
Recently, increasing attempts have been made to understand how plant genes function in natura. In this context, transcriptional profiles represent plant physiological status in response to environmental stimuli. Herein, we combined high-throughput RNA-Seq with insect survey data on 19 accessions of Arabidopsis thaliana grown at a field site in Switzerland. We found that genes with the gene ontology (GO) annotations of "glucosinolate biosynthetic process" and "response to insects" were most significantly enriched, and the expression of these genes was highly variable among plant accessions. Nearly half of the total expression variation in the glucosinolate biosynthetic genes (AOPs, ESM1, ESP, and TGG1) was explained by among-accession variation. Of these genes, the expression level of AOP3 differed among Col-0 accession individuals depending on the abundance of the mustard aphid (Lipaphis erysimi). We also found that the expression of the major cis-Jasmone activated gene CYP81D11 was positively correlated with the number of flea beetles (Phyllotreta striolata and Phyllotreta atra). Combined with the field RNA-Seq data, bioassays confirmed that AOP3 was up-regulated in response to attack by mustard aphids. The combined results from RNA-Seq and our ecological survey illustrate the feasibility of using field transcriptomics to detect an inducible defense, providing a first step towards an in natura understanding of biotic interactions involving phenotypic plasticity.
Feeding experiment using uniformly (13)C-labeled alpha-linolenic acid supports the involvement of the decarboxylation mechanism to produce cis-jasmone in Lasiodiplodia theobromae.[Pubmed:31342844]
Biosci Biotechnol Biochem. 2019 Dec;83(12):2190-2193.
In our previous report, it was found that Lasiodiplodia theobromae produced cis-Jasmone via partially utilizing the biosynthetic pathway of JA. A feeding experiment using uniformly (13)C-labeled alpha-linolenic acid, which was added to the culture media of the fungus, strongly supported that the fungus produced CJ via the decarboxylation step of the biosynthetic pathway.
Chemical Eustress Elicits Tailored Responses and Enhances the Functional Quality of Novel Food Perilla frutescens.[Pubmed:30621323]
Molecules. 2019 Jan 6;24(1). pii: molecules24010185.
Consumer demand for fresh and functional horticultural products is on the rise. Perilla frutescens, L. Britt (Lamiaceae) is a potential specialty/niche crop for consumption and therapeutic uses with high contents of phenolic and volatile compounds. Plant growth, mineral composition, polyphenol profile and aroma volatile components of two perilla genotypes in response to salinity (non-salt control, 10, 20 or 30 mM NaCl) applied as chemical eustressor were assessed. Salinity suppressed growth and yield of both genotypes, although the red-pigmented genotype was less sensitive than the green-pigmented one. Mild (10 mM NaCl) and moderate (20 and 30 mM NaCl) salinity suppressed foliar potassium, magnesium, nitrate and chlorophyll a concentrations of both genotypes and increased the levels of rosmarinic acid, total polyphenols and target aroma volatile components. Green perilla showed higher yield and biomass production and higher content of protein, dry matter, calcium, magnesium, perilla ketone and cis-Jasmone, whereas red perilla exhibited higher content of potassium, chlorophyll a, rosmarinic acid, total polyphenols, perilla aldehyde and benzaldehyde. Our findings support that chemical eustressors such as mild to moderate salinity offer valuable means to manipulate phytochemical and aroma profiles.
Plant defense elicitors: plant fitness versus wheat stem sawfly.[Pubmed:30402358]
PeerJ. 2018 Nov 1;6:e5892.
The wheat stem sawfly (WSS), Cephus cinctus Norton, is an important wheat pest in the Northern Great Plains of the USA. No single control measure effectively suppresses WSS damage. This study provides information on the effects on the WSS adult settling preference behavior on wheat plants under laboratory conditions from treatment with both synthetic plant defense elicitors (Actigard((R)) and cis-Jasmone) and a botanical insecticide (Azadirachtin((R))). In addition, field experiments were performed to determine whether these chemicals impact the WSS fitness (larval mortality and larval body weight), winter wheat plant fitness (infestation, stem lodging, yield, and quality), adult population of WSS and Bracon spp., and larval parasitism levels. Our lab results showed that there were no significant differences in adult settling behavior on plants exposed separately to each chemical and control. In contrast, when adults were exposed simultaneously to treated and untreated plants, there was a significant reduction in the percentage of adults settling on Actigard((R)) and Azadirachtin((R)) treated plants compared to plants sprayed with water in the same cage. However, in field situations, regardless of application timing and field location, none of the chemicals significantly reduced adult population or stems damage. The exception was two times applications of Actigard((R)) had significantly lower WSS infested stem damage levels at 30 days after initial treatment applications at Knees and 50 days at Choteau locations compared to control, but without effect at the Conrad location. The field study indicated that two times applications of Actigard((R)) significantly increased diapausing larval mortality percentages and lowered stem lodging levels compared to untreated controls at Knees and Choteau locations, while no effects at Conrad location. Larval body weight was significantly lower in plots treated with Actigard((R)) at Knees and Conrad, but no effects at Choteau. No significant differences were found in wheat yield and quality in plots treated with chemicals and controls at any location. Bracon spp. adult population and parasitism levels were not negatively affected by the use of chemicals. In conclusion, this study offers insights on what treatments should be emphasized in more detail despite variable findings.
Novel Hydroxy- and Epoxy-cis-Jasmone and Dihydrojasmone Derivatives Affect the Foraging Activity of the Peach Potato Aphid Myzus persicae (Sulzer) (Homoptera: Aphididae).[Pubmed:30223586]
Molecules. 2018 Sep 15;23(9). pii: molecules23092362.
Jasmonates show great potential in sustainable agriculture due to their various roles in natural mechanisms of plant defense, and because they are non-toxic, non-mutagenic, and easily metabolized. The aim of the study was to explore structure(-)activity relationships of dihydrojasmone, cis-Jasmone, and their derivatives at the plant(-)aphid interface. We focused on the behavioral responses of aphids, following the exogenous application of natural jasmonates and their derivatives to the host plants. Aphid probing behavior was examined using an electrical penetration graph technique (EPG). The chemoenzymatic transformation of cis-Jasmone and the activity of two new derivatives are described. The application of cis-Jasmone, dihydrojasmone, the hydroxyderivatives, epoxyderivatives, and alkyl-substituted delta-lactones hindered the foraging activity of Myzus persicae (Sulz.) (Hemiptera: Aphididae) during early stages of probing at the level of non-phloem tissues. The application of saturated bicyclic epoxy-delta-lactone enhanced plant acceptance by M. persicae. Jasmonate derivatives containing a hydroxy group, especially in correlation with a lactone ring, were more active than natural compounds and other derivatives studied. Jasmonates of the present study are worth considering as elements of sustainable aphid control as components of the "push(-)pull" strategy.
Synthesis of Diketones, Ketoesters, and Tetraketones by Electrochemical Oxidative Decarboxylation of Malonic Acid Derivatives: Application to the Synthesis of cis-Jasmone.[Pubmed:30208277]
J Org Chem. 2018 Oct 5;83(19):12044-12055.
Disubstituted malonic acid derivatives are smoothly converted into diketones and ketoesters in good to excellent yield (68% to 91%) under electrochemical conditions. The scope can be extended to transform trisubstituted bis-malonic acids into tetraketones in 77% to 86% yield. The new method was applied to the total synthesis of cis-Jasmone.
Plant Biomarker Recognition by Molecular Imprinting Based Localized Surface Plasmon Resonance Sensor Array: Performance Improvement by Enhanced Hotspot of Au Nanostructure.[Pubmed:30074768]
ACS Sens. 2018 Aug 24;3(8):1531-1538.
Detection of plant volatile organic compounds (VOCs) enables monitoring of pests and diseases in agriculture. We previously revealed that a localized surface plasmon resonance (LSPR) sensor coated with a molecularly imprinted sol-gel (MISG) can be used for cis-Jasmone vapor detection. Although the selectivity of the LSPR sensor was enhanced by the MISG coating, its sensitivity was decreased. Here, gold nanoparticles (AuNPs) were doped in the MISG to enhance the sensitivity of the LSPR sensor through hot spot generation. The size and amount of AuNPs added to the MISG were investigated and optimized. The sensor coated with the MISG containing 20 muL of 30 nm AuNPs exhibited higher sensitivity than that of the sensors coated with other films. Furthermore, an optical multichannel sensor platform containing different channels that were bare and coated with four types of MISGs was developed to detect plant VOCs in single and binary mixtures. Linear discriminant analysis, k-nearest neighbor (KNN), and naive Bayes classifier approaches were used to establish plant VOC identification models. The results indicated that the KNN model had good potential to identify plant VOCs quickly and efficiently (96.03%). This study demonstrated that an LSPR sensor array coated with a AuNP-embedded MISG combined with a pattern recognition approach can be used for plant VOC detection and identification. This research is expected to provide useful technologies for agricultural applications.
An odorant receptor mediates the attractiveness of cis-jasmone to Campoletis chlorideae, the endoparasitoid of Helicoverpa armigera.[Pubmed:30058747]
Insect Mol Biol. 2019 Feb;28(1):23-34.
Parasitic wasps rely on olfaction to locate their hosts in complex chemical environments. Odorant receptors (ORs) function together with well-conserved odorant coreceptors (ORcos) to determine the sensitivity and specificity of olfactory reception. Campoletis chlorideae (Hymenoptera: Ichneunmonidae) is a solitary larval endoparasitoid of the cotton bollworm, Helicoverpa armigera, and some other noctuid species. To understand the molecular basis of C. chlorideae's olfactory reception, we sequenced the transcriptome of adult male and female heads (including antennae) and identified 211 OR transcripts, with 95 being putatively full length. The tissue expression profiles, as assessed by reverse-transcription PCR, showed that seven ORs were expressed only or more highly in female antennae. Their functions were analysed using the Xenopu slaevis oocyte expression system and two-electrode voltage-clamp recordings. CchlOR62 was tuned to cis-Jasmone, which was attractive to female C. chlorideae adults and H. armigera larvae in the subsequent behavioural assays. Further bioassays using caged plants showed that the parasitism rate of H. armigera larvae by C. chlorideae on cis-Jasmone-treated tobacco plants was higher than on the control plants. Thus, cis-Jasmone appears to be an important infochemical involved in the interactions of plants, H. armigera and C. chlorideae, and CchlOR62 mediates the attractiveness of cis-Jasmone to C. chlorideae.


