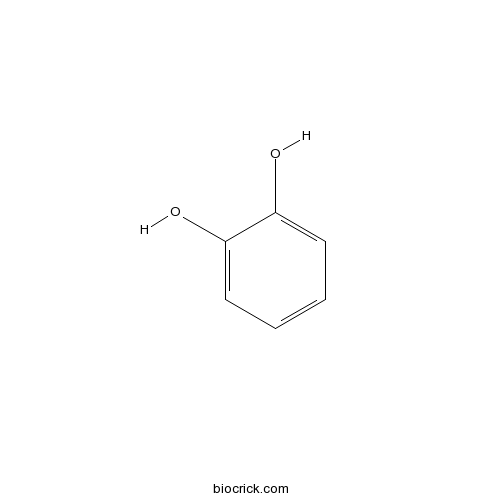
A benzenediol comprising of a benzene core carrying two hydroxy substituents ortho to each other.
1,2-Dihydroxybenzene (catechol) can induce spontaneous convulsive activity in the anaesthetized mouse.[1]
1,2-Dihydroxybenzene can produce myoclonic jerks in the rat.[2]
1,2-Dihydroxybenzene-containing compounds bind to and cap cysteine residues of tau and prevent its aggregation by hindering interactions between tau molecules, further, we show that orally administered DL-isoproterenol, an adrenergic receptor agonist whose skeleton includes 1,2-dihydroxybenzene and which penetrates the brain, reduces the levels of detergent-insoluble tau, neuronal loss and reverses neurofibrillary tangle-associated brain dysfunction; thus, compounds that target the cysteine residues of tau may prove useful in halting the progression of Alzheimer’s disease and other tauopathies.[3]
English website: 1,2-Benzenediol
Japanese website: 1,2-Benzenediol
Chinese website: 1,2-Benzenediol
[1] Angel A, Clarke K A, Dewhurst D G. Brit J Pharmacol, 1977, 61(3):433-9.
[2] Angel A, Lemon R N. Electroencephalography & Clinical Neurophysiology, 1973, 35(6):589-601.
[3] Milosz C. Nature Commun, 2015, 6(4):141-7.
[4] Ruan G H. Environmental and health magazine, 2002, 19 (1) : 64-5.