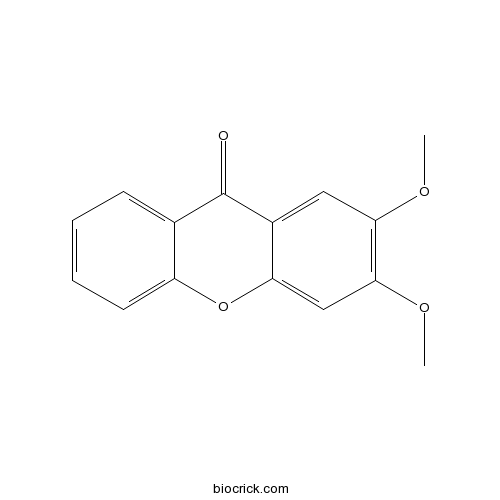
2,3-DimethoxyxanthoneCAS# 42833-49-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 42833-49-8 | SDF | Download SDF |
| PubChem ID | 253955 | Appearance | Powder |
| Formula | C15H12O4 | M.Wt | 256.25 |
| Type of Compound | Xanthones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2,3-dimethoxyxanthen-9-one | ||
| SMILES | COC1=C(C=C2C(=C1)C(=O)C3=CC=CC=C3O2)OC | ||
| Standard InChIKey | FWCWUPRCWDSQEN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H12O4/c1-17-13-7-10-12(8-14(13)18-2)19-11-6-4-3-5-9(11)15(10)16/h3-8H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

2,3-Dimethoxyxanthone Dilution Calculator

2,3-Dimethoxyxanthone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9024 mL | 19.5122 mL | 39.0244 mL | 78.0488 mL | 97.561 mL |
| 5 mM | 0.7805 mL | 3.9024 mL | 7.8049 mL | 15.6098 mL | 19.5122 mL |
| 10 mM | 0.3902 mL | 1.9512 mL | 3.9024 mL | 7.8049 mL | 9.7561 mL |
| 50 mM | 0.078 mL | 0.3902 mL | 0.7805 mL | 1.561 mL | 1.9512 mL |
| 100 mM | 0.039 mL | 0.1951 mL | 0.3902 mL | 0.7805 mL | 0.9756 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Hydroxytyrosol 4-O-glucoside
Catalog No.:BCN9312
CAS No.:54695-80-6
- 2,5,2'-Trihydroxy-4-methoxybenzophenone
Catalog No.:BCN9311
CAS No.:202463-52-3
- Creoside III
Catalog No.:BCN9310
CAS No.:1038602-12-8
- Juncatrin A
Catalog No.:BCN9309
CAS No.:2265925-12-8
- Ethyl β-D-xylopyranoside
Catalog No.:BCN9308
CAS No.:6743-62-0
- 3,5,3'-Trihydroxystilbene
Catalog No.:BCN9307
CAS No.:150258-84-7
- Simulanol
Catalog No.:BCN9306
CAS No.:500574-38-9
- 3,5,3'-Trihydroxybibenzyl
Catalog No.:BCN9305
CAS No.:86630-23-1
- Taxilluside A
Catalog No.:BCN9304
CAS No.:1661914-46-0
- n-Butyl β-D-fructofuranoside
Catalog No.:BCN9303
CAS No.:80971-60-4
- ent-13,16β,17-Trihydroxykauran-19-oic acid
Catalog No.:BCN9302
CAS No.:142543-30-4
- 1,6-Dimethyl-4,5-dihydropyrene-2,7-diol
Catalog No.:BCN9301
CAS No.:2255331-28-1
- Aspyridone A
Catalog No.:BCN9314
CAS No.:935863-26-6
- 10-Hydroxymajoroside
Catalog No.:BCN9315
CAS No.:259753-12-3
- Flazin
Catalog No.:BCN9316
CAS No.:100041-05-2
- 3-epi-Isocucurbitacin B
Catalog No.:BCN9317
CAS No.:89647-62-1
- 10-Hydroxyligstroside
Catalog No.:BCN9318
CAS No.:35897-94-0
- Jinflexin D
Catalog No.:BCN9319
CAS No.:2055155-78-5
- Dicliripariside A
Catalog No.:BCN9320
CAS No.:491613-65-1
- Giffonin P
Catalog No.:BCN9321
CAS No.:1830306-93-8
- Hymexelsin
Catalog No.:BCN9322
CAS No.:117842-09-8
- Leeaoside
Catalog No.:BCN9323
CAS No.:1015063-56-5
- Phoyunnanin E
Catalog No.:BCN9324
CAS No.:886747-60-0
- Icterogenin
Catalog No.:BCN9325
CAS No.:561-47-7
Antistaphylococcal Prenylated Acylphoroglucinol and Xanthones from Kielmeyera variabilis.[Pubmed:26900954]
J Nat Prod. 2016 Mar 25;79(3):470-6.
Bioactivity-guided fractionation of the EtOH extract of the branches of Kielmeyera variabilis led to the isolation of a new acylphoroglucinol (1), which was active against all the MRSA strains tested herein, with pronounced activity against strain EMRSA-16. Compound 1 displayed an MIC of 0.5 mg/L as compared with an MIC of 128 mg/L for the control antibiotic norfloxacin. The structure of the new compound was elucidated by 1D and 2D NMR spectroscopic analysis and mass spectrometry, and experimental and calculated ECD were used to determine the absolute configurations. The compounds beta-sitosterol (2), stigmasterol (3), ergost-5-en-3-ol (4), and osajaxanthone (5) also occurred in the n-hexane fraction. The EtOAc fraction contained nine known xanthones: 3,6-dihydroxy-1,4,8-trimethoxyxanthone (6), 3,5-dihydroxy-4-methoxyxanthone (7), 3,4-dihydroxy-6,8-dimethoxyxanthone (8), 3,4-dihydroxy-2-methoxyxanthone (9), 5-hydroxy-1,3-dimethoxyxanthone (10), 4-hydroxy-2,3-Dimethoxyxanthone (11), kielcorin (12), 3-hydroxy-2-methoxyxanthone (13), and 2-hydroxy-1-methoxyxanthone (14), which showed moderate to low activity against the tested MRSA strains.
[Chemical Constituents of Ethyl Acetate Fraction from Hypericum ascyron].[Pubmed:30080367]
Zhong Yao Cai. 2016 Feb;39(2):322-5.
Objective: To study the chemical consituents of Hypericum ascyron. Methods: The constituents were isolated and purified by chromatography on silica gel; the structure of the compound was determined by MS and NMR spectral analysis. Results: On the basis of spectroscopic analysis and comparison with the reported data, they were identified as hyperoside( 1),hypercalin B( 2),hypercalin C( 3),1,7-dihydroxyxanthone( 4),2,3-Dimethoxyxanthone( 5),1-hydroxy-7-methoxyxanthone( 6),rutin( 7),kaempferol( 8),toxyloxanthone B( 9),quercetin( 10),quercitrin( 11),beta-daucosterol( 12) and beta-sitosterol( 13). Conclusion: Compounds 2,3,6 and 9 are obtained from this plant for the first time.
Anti-ulcer xanthones from the roots of Hypericum oblongifolium Wall.[Pubmed:24685505]
Fitoterapia. 2014 Jun;95:258-65.
Three new xanthones, hypericorin C (1), hypericorin D (2) and 3,4-dihydroxy-5-methoxyxanthone (3), along with eight known compounds; 2,3-Dimethoxyxanthone (4), 3,4-dihydroxy-2-methoxyxanthone (5), 3,5-dihydroxy-1-methoxyxanthone (6), 3-acetylbetulinic acid (7), 10H-1,3-dioxolo[4,5-b]xanthen-10-one (8), 3-hydroxy-2-methoxyxanthone (9), 3,4,5-trihydroxyxanthone (10) and betulinic acid (11) were isolated from the roots of Hypericum oblongifolium. The structures of the new compounds 1, 2 and 3 were deduced by spectroscopic techniques [ESI MS, (1)H NMR, (13)C NMR, and 2D NMR (HMQC, HMBC, COSY and NOESY)]. The entire series of compounds were evaluated for anti-ulcer activity.
Role of gastric mucus secretion, oxinitrergic system and sulfhydryl groups on the gastroprotection elicited by Polygala cyparissias (Polygalaceae) in mice.[Pubmed:23600395]
J Pharm Pharmacol. 2013 May;65(5):767-76.
OBJECTIVES: This study has aimed to assess the mechanisms of action for the gastroprotective effect of the acetone extract (PCAE) and methanol fraction (PCMF) of Polygala cyparissias, as well as to evaluate the activity of 1,3,6,8-tetrahydroxy-2,7-dimethoxyxanthone (1), 1,7-dihydroxy-2,3-Dimethoxyxanthone (2) and astragalin (3). METHODS: Gastric secretion and mucus content were determined by pylorus ligation in mice. Nitric oxide (NO) and sulfhydryl group participation were observed by the pretreatment of mice with L-NAME or NEM. Acute ulcer was induced by ethanol/HCl and chronic ulcer by acetic acid. Anti-Helicobacter pylori activity was evaluated by the agar solid dilution assay. KEY FINDINGS: Neither PCAE nor PCMF had the ability to reduce H(+) concentration. However, both of them enhanced mucus secretion. PCAE demonstrated its gastroprotection in a NO-dependent manner, while PCMF exerted the activity depending on the sulfhydryl group. In chronic ulcer, the curative ratios for the PCAE and PCMF were 67.5 and 58.4%, respectively. No effect over H. pylori was detected. Compounds 1, 2 and 3 were able to reduce lesions in the order of 79.6, 73.8 and 67.6%, respectively. CONCLUSIONS: The data suggested that PCAE and PCMF displayed antiulcer activity due to different mechanisms and with the participation of phenolic compounds obtained from the plant.
Anti-inflammatory xanthones from the twigs of Hypericum oblongifolium wall.[Pubmed:21870324]
Planta Med. 2011 Dec;77(18):2013-8.
Two new xanthonolignoids, hypericorin A (1) and hypericorin B (2), along with five known new source compounds, a xanthonolignoid, kielcorin (3), 4-hydroxy-2,3-Dimethoxyxanthone (4), 3,4,5-trihydroxyxanthone (5), 1,3-dihydroxy-5-methoxyxanthone ( 6) and 1,3,7-trihydroxyxanthone (7), were isolated from the stems (twigs) of Hypericum oblongifolium Wall. The structures of the new compounds were deduced on the basis of spectroscopic techniques (EI-MS, HREI-MS, (1)H NMR, (13)C NMR, HMQC, HMBC, and NOESY). We also report herein for the first time the single crystal X-ray structure of compound 6. Compounds 1- 7 were screened for their IN VITRO anti-inflammatory (respiratory burst) inhibiting activities using isolated human neutrophils; compounds 1, 2, 3, 5, and 7 showed significant activities (IC (50) = 816.23 +/- 73.30, 985.20 +/- 55.80, 965.21 +/- 65.80, 907.20 +/- 50.80, 975.20 +/- 81.10 microM, respectively), compound 6 showed moderate activity (IC (50) = 2500.85 +/- 50.50 microM), while compound 4 was totally inactive at 1000 microg/mL as compared to the positive control used, indomethacin (IC (50) = 757.99 +/- 5.90 microM), and aspirin (IC (50) = 279.44 +/- 4.40 microM). Compound 4 was also inactive in comparison with other tested Hypericum compounds.
Chemical constituents of Polygala tenuifolia roots and their inhibitory activity on lipopolysaccharide-induced nitric oxide production in BV2 microglia.[Pubmed:21740104]
J Enzyme Inhib Med Chem. 2012 Feb;27(1):1-4.
A methanolic extract of the roots of Polygala tenuifolia (Polygalaceae) significantly attenuated nitric oxide (NO) production in lipopolysaccharide (LPS)-stimulated BV2 microglia cells. Five xanthones, 1-hydroxy-7-methoxyxanthone (1), 3,6-dihydroxy-1,2,7-trimethoxyxanthone (2), 1,3,6-trihydroxy-2,7-dimethoxyxanthone (3), 1,7-dihydroxy-2,3-Dimethoxyxanthone (4) and 1,7-dihydroxy-3-methoxyxanthone (5), and five phenylpropanoids, 4-hydroxy-3-methoxypropiophenone (6), methyl 4-hydroxy-3-methoxycinnamic acid (7), 3,4,5-trimethoxycinnamic acid (8), 4-methoxycinnamic acid (9) and beta-d-(3-O-sinapoyl) fructofuranosyl-alpha-d-(6-O-sinapoyl)glucopyranoside (10), were isolated from CHCl(3) fraction using bioactivity-guided fractionation. Among these compounds, compounds 1, 2, 4, 5 and 7 showed significant inhibitory effects on LPS-induced NO production in BV2 microglia cells at the concentration ranging from 10.0 to 100.0 muM.
Bioactive xanthones from the roots of Hypericum perforatum (common St John's wort).[Pubmed:21218475]
J Sci Food Agric. 2011 Feb;91(3):428-34.
BACKGROUND: Extracts of Hypericum perforatum L. (common St John's wort; Hypericaceae) are sold as phytopharmaceuticals and herbal supplements to treat mild to moderate depression and as food additives. Extensively cultivated in Europe, plants can be infected by anthracnose (Colletotrichum gloeosporioides), a virulent fungal pathogen that causes tissue necrosis and dramatically decreases crop value. Such infections triggered the production of new secondary metabolites, specifically xanthones, in cell culture experiments. RESULTS: Bioassay-guided fractionation of H. perforatum root extracts, testing for growth inhibition of plant pathogenic fungi from the genera Colletotrichum, Botrytis, Fusarium and Phomopsis, was performed. In vitro anti-inflammatory activity through inhibition of COX-1, COX-2 and 5-LOX-catalyzed LTB(4) formation was also evaluated. Extracts were analyzed by various chromatographic means and structure elucidation was performed using data from nuclear magnetic resonance and mass spectrometry. CONCLUSION: Researchers have previously described constituents from the aerial parts of this species, but few reports describe secondary metabolites found in underground parts, of particular interest because the lower stem and upper root are often sites of fungal infection. This work resulted in the isolation of three xanthones: 1,6-dihydroxy-5-methoxy-4',5'-dihydro-4',4',5'-trimethylfurano-(2',3':3,4)-xantho ne; 4,6-dihydroxy-2,3-Dimethoxyxanthone; and cis-kielcorin, one of which possessed novel bioactivity against species of Phomopsis and inhibited 5-LOX-mediated LTB(4) formation.
Xanthones from Polygala alpestris (Rchb.).[Pubmed:18998397]
Z Naturforsch C J Biosci. 2004 May-Jun;59(5-6):335-8.
Bioactivity-guided fractionation of Polygala alpestris L. (Rchb.) extracts led to the identification of two new xanthones, 1,3,7-trihydroxy-2,6-dimethoxyxanthone (1) and 2,3-methylenedioxy-4,7-dihydroxyxanthone (2). In addition five known compounds 3,4-dimethoxy-1,7-dihydroxyxanthone (3), 1,3-dihydroxy-7-methoxyxanthone (4), 1,7-dihydroxy-2,3-Dimethoxyxanthone (5), 3',6-O-disinapoyl sucrose (6) and 3',5'-dimethoxybiphenyl-4-olo (7) were isolated. The structures of the isolated compounds were established by means of high resolution mass spectrometry, mono- and bi-dimensional NMR spectroscopy. All isolated compounds were tested for cytotoxic activity against three tumor cell lines (LoVo, HL-60, K 562).
Xanthones from stems of Hypericum chinense.[Pubmed:17202694]
Chem Pharm Bull (Tokyo). 2007 Jan;55(1):19-21.
Six new xanthones, 4,6-dihydroxy-2,3-Dimethoxyxanthone (1), 2,6-dihydroxy-3,4-dimethoxyxanthone (2), 6-hydroxy-2,3,4-trimethoxyxanthone (3), 3,6-dihydroxy-1,2-dimethoxyxanthone (4), 4,7-dihydroxy-2,3-Dimethoxyxanthone (5), and 3,7-dihydroxy-2,4-dimethoxyxanthone (6) were isolated from the stems of Hypericum chinense, together with four known xanthones. Their structures were established based on spectroscopic studies.
Xanthones from Hypericum chinense.[Pubmed:16884745]
Phytochemistry. 2006 Oct;67(19):2146-51.
Six xanthones, 1,3,7-trihydroxy-2-(2-hydroxy-3-methyl-3-butenyl)-xanthone (1), 1,7-dihydroxy-2,3-[2''-(1-hydroxy-1-methylethyl)-dihydrofurano]-xanthone (2), 1,3,7-trihydroxy-5-methoxyxanthone (3), 1,7-dihydroxy-5,6-dimethoxyxanthone (4), 4,5-dihydroxy-2,3-Dimethoxyxanthone (5), 1,3-dihydroxy-2,4-dimethoxyxanthone (6) and 21 known xanthones were isolated from the leaves and stems of Hypericum chinense. Their structures were established based on spectroscopic studies.
Microbial O-demethylation, hydroxylation, sulfation, and ribosylation of a xanthone derivative from Halenia elliptica.[Pubmed:16724847]
J Nat Prod. 2006 May;69(5):811-4.
1-Hydroxy-2,3,5-trimethoxyxanthone (1), one of the major xanthone derivatives isolated from Halenia elliptica, was biotransformed by two fungi, Trichothecium roseum and Paecilomyces marquandii. Transformation of 1 by T. roseumgave 1,5-dihydroxy-2,3-Dimethoxyxanthone (2), 5-O-sulfate-1-hydroxy-2,3-Dimethoxyxanthone (3), 5-O-sulfate-1-hydroxy-2,3,7-trimethoxyxanthone (4), 5-O-beta-ribofuranosyl-1-hydroxy-2,3-Dimethoxyxanthone (5), and 1,5,6-trihydroxy-2,3-Dimethoxyxanthone (6). Compound 2 was also formed by P. marquandii. The structures of the isolated compounds were elucidated by spectroscopic analyses. Among the five microbial-converted compounds, 3, 4, 5, and 6 are new compounds.
[Chemical constituents in roots of Polygala fallax and their anti-oxidation activities in vitro].[Pubmed:16110862]
Zhongguo Zhong Yao Za Zhi. 2005 Jun;30(11):827-30.
OBJECTIVE: To study the chemical constituents in roots of P. fallax and their anti-oxidation activities in vitro. METHOD: Column chromatographic techniques were employed for isolation and purification of chemical constituents of the plant. The structures were elucidated on the basis of the spectral evidence and the physical and chemical character. The isolated compounds were screened with four anti-oxidation models in vitro. RESULT: Seven xanthones, 1,7-dihydroxy-2,3-methylenedioxyxanthone (1), 1-methoxy-2,3-methylenedioxyxanthone (2), 3-hydroxy-1,2-dimethoxyxanthone (3), 1,6,7-trihydroxy-2,3-Dimethoxyxanthone (4), 7-hydroxy-1-methoxy-2,3-methylenedioxyxanthone (5), 1,3-dihydroxy-2-methoxyxanthone (6) and 1,3,7-trihydroxy-2-methoxyxanthone (7), were isolated from the roots of P. fallax. And compounds 1 - 7 showed different anti-oxidation activities in the different pharmacological models. CONCLUSION: Compounds 2, 3, 5 and 7 were isolated from this plant for the first time. Xanthones from this plant showed anti-oxidation activities. The pharmacological activities of the pure compounds from this plant were also reported for the first time.
Two xanthones from Polygala paniculata and confirmation of the 1-hydroxy-2,3,5-trimethoxy-xanthone at trace level by HRGC-MS.[Pubmed:12939033]
Z Naturforsch C J Biosci. 2003 Jul-Aug;58(7-8):490-4.
Polygala paniculata L. yielded the xanthones 1-hydroxy-5-methoxy-2,3-methylenedioxyxanthone (1) and 1,5-dihydroxy-2,3-Dimethoxyxanthone (2), together with coumarin murragatin and flavonol rutin. Their structures were established by chemical and spectroscopic methods (EIMS, IR, 1H and 13C NMR, NOE difference spectroscopy). By posterior analysis of an apolar crude extract using high resolution gas chromatography coupled to mass spectrometry (HRGC-MS) it was possible to characterize two sterol (spinasterol and delta25-spinasterol) and the minor 1-hydroxy-2,3,5-trimethoxyxanthone (3). Thus, the xanthone 3 was confirmed through of co-injection HRGC-MS of the respective extract with a certified standard obtained by methylation of 2 with diazomethane.


