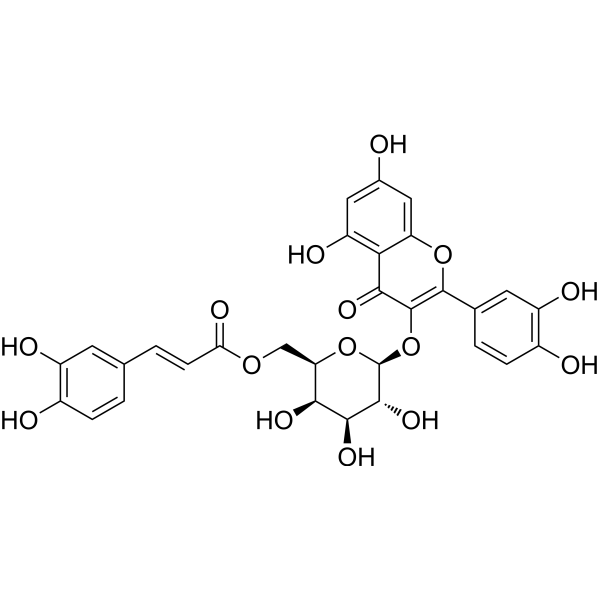
3-HydroxyirisquinoneCAS# 100205-29-6 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 100205-29-6 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C24H38O4 | M.Wt | 626.52 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | 5-Hydroxyirisquinone | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

3-Hydroxyirisquinone Dilution Calculator

3-Hydroxyirisquinone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5961 mL | 7.9806 mL | 15.9612 mL | 31.9224 mL | 39.903 mL |
| 5 mM | 0.3192 mL | 1.5961 mL | 3.1922 mL | 6.3845 mL | 7.9806 mL |
| 10 mM | 0.1596 mL | 0.7981 mL | 1.5961 mL | 3.1922 mL | 3.9903 mL |
| 50 mM | 0.0319 mL | 0.1596 mL | 0.3192 mL | 0.6384 mL | 0.7981 mL |
| 100 mM | 0.016 mL | 0.0798 mL | 0.1596 mL | 0.3192 mL | 0.399 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Hancinone D
Catalog No.:BCX1777
CAS No.:104973-90-2
- Salvileucalin A
Catalog No.:BCX1776
CAS No.:1053241-84-1
- Arundanine
Catalog No.:BCX1775
CAS No.:618852-71-4
- (±)-7-epi-Kadsurenone
Catalog No.:BCX1774
CAS No.:245648-20-8
- 6,7-Dihydrosalviandulin E
Catalog No.:BCX1773
CAS No.:1983982-37-1
- Dugesin B
Catalog No.:BCX1772
CAS No.:760973-66-8
- Quercetin 3-O-β-D-xylofuranosyl-(1→2)-β-D-galactopyranoside
Catalog No.:BCX1771
CAS No.:1181635-91-5
- (7R,8S,8′R)-4,7-Dihydroxy-3,3′,4′-trimethoxyl-9-oxo dibenzylbutyrolactone lignan-4-O-β-D-glucopyrano...
Catalog No.:BCX1770
CAS No.:1807808-90-7
- Salvileucantholide
Catalog No.:BCX1769
CAS No.:158994-33-3
- Dugesin C
Catalog No.:BCX1768
CAS No.:1403505-97-4
- Poricoic acid H
Catalog No.:BCX1767
CAS No.:415724-85-5
- Donasine
Catalog No.:BCX1766
CAS No.:1017237-81-8
- Quercetin 3-O-(6′′-caffeoyl)-β-D-galactopyranoside
Catalog No.:BCX1779
CAS No.:316354-12-8
- Quercetin 3-galactosyl(1→2)rhamnoside
Catalog No.:BCX1780
CAS No.:189135-70-4
- 6,7-Dehydrodugesin A
Catalog No.:BCX1781
CAS No.:1542141-27-4
- Qianhucoumarin B
Catalog No.:BCX1782
CAS No.:152615-14-0
- Qianhucoumarin C
Catalog No.:BCX1783
CAS No.:152615-15-1
- 6-Methoxykaempferol 3-O-β-D-glucopyranosyl-(1 →2)-β-D-glucopyranosyl-7-O-β-D- glucopyranoside
Catalog No.:BCX1784
CAS No.:2364631-99-0
- 6-Methoxykaemferol-3-O-β-D-glucosyl (1'″→2″)-β-D-glucopyranosyl-(6″″-(E)-caffeoyl) -7-O-β-D-glucopyr...
Catalog No.:BCX1785
CAS No.:1005416-14-7
- 3-O-Methylquercetin 7-O-β-D-glucopyranosyl-4′-O-β-D-glucopyranoside
Catalog No.:BCX1786
CAS No.:47858-32-2
- Pedunculosumoside F
Catalog No.:BCX1787
CAS No.:1283600-08-7
- Ketologanin
Catalog No.:BCX1788
CAS No.:152-91-0
- Perilla ketone
Catalog No.:BCX1789
CAS No.:553-84-4
- Transilin
Catalog No.:BCX1790
CAS No.:26931-68-0
Metabolomic Responses of Lettuce (Lactuca sativa) to Allelopathic Benzoquinones from Iris sanguinea Seeds.[Pubmed:36961423]
J Agric Food Chem. 2023 Apr 5;71(13):5143-5153.
Weed management is important in modern crop protection. Chemical weed control using synthetic herbicides, however, suffers from resistance and ecotoxicity. Metabolomic investigation of allelopathy (or allelochemicals) may provide novel alternatives to synthetic herbicides. This study aimed to investigate the detailed metabolomic responses of plants to allelochemicals in Iris seed extracts. The seed extracts of Iris sanguinea showed the strongest growth inhibitory activity against alfalfa, barnyard grass, lettuce, and mustard. 3-Hydroxyirisquinone (3-[10(Z)-heptadecenyl]-2-hydroxy-5-methoxy-1,4-benzoquinone) was isolated as a major allelochemical from I. sanguinea seeds through bioassay-guided fractionation. The compound inhibited the growth of shoots and roots by browning root tips. Discriminant analysis identified 33 differentially regulated lettuce metabolites after treatment with 3-Hydroxyirisquinone (3HIQ). Metabolic pathway analysis revealed that several metabolic pathways, including aromatic amino acid biosynthesis and respiratory pathways, were affected by the compounds. Differential responses of membrane lipids (accumulation of unsaturated fatty acids) and extensive formation of reactive oxygen species were observed in root tissues following treatment with 3HIQ. Overall, alkylbenzoquinone from I. sanguinea induced extensive metabolic modulation, oxidative stress, and growth inhibition. The metabolomic responses to allelochemicals may provide fundamental information for the development of allelochemical-based herbicides.
A new belamcandaquinone from the seeds of Iris bungei Maxim.[Pubmed:21820495]
Fitoterapia. 2011 Oct;82(7):1137-9.
A novel dimeric 1,4-benzoquinone and resorcinol derivative, Belamcandaquinone N (1), and two known compounds, 3-Hydroxyirisquinone (2) and 5-[(Z)-10-heptadecenyl] resorcinol (3), were isolated from the seeds of Iris bungei Maxim. Their structures were elucidated by spectroscopic methods and comparing with literature data of known compounds. These compounds showed remarkable cytotoxic activity against RM-1 cell lines.
Antioxidant and anticholinesterase constituents from the petroleum ether and chloroform extracts of Iris suaveolens.[Pubmed:20830698]
Phytother Res. 2011 Apr;25(4):522-9.
The aim of this study was to investigate the phytochemical, antioxidant and anticholinesterase activities of Iris suaveolens. After determining total phenolic and flavonoid contents of the petroleum ether, chloroform and methanol extracts prepared from the rhizomes, the antioxidant capacity of the extracts was established using beta-carotene-linoleic acid and CUPRAC methods. The chloroform extract which was rich in phenolic content exhibited the highest inhibition of lipid peroxidation in the beta-carotene-linoleic acid system, and the best cupric reducing antioxidant capacity among the tested extracts. The petroleum ether extract indicated moderate anticholinesterase activity while the chloroform extract revealed significant butyrylcholinesterase inhibitory activity (75.03 +/- 1.29%). Spectroscopic methods were used for the structural elucidation of the compounds (1-13) isolated from the petroleum ether and chloroform extracts. Coniferaldehyde (6), having the highest antioxidant activity in the beta-carotene-linoleic acid assay at 25 and 50 microg/mL, demonstrated also the best effect in the CUPRAC method among the tested compounds (1-12). 3-Hydroxyirisquinone (10) showed the best anticholinesterase activity among the tested compounds (1-4, 6-12), and coniferaldehyde exhibited almost the same butyrylcholinesterase inhibitory activity (82.60 +/- 2.33%) as galantamine (86.26 +/- 0.66%).
Two new quinones from Iris bungei.[Pubmed:10823714]
Chem Pharm Bull (Tokyo). 2000 May;48(5):738-9.
Two new benzoquinone derivatives, bungeiquinone (1) and dihydrobungeiquinone (2), and two known derivatives, 3-Hydroxyirisquinone (3) and 3-hydroxydihydroirisquinone (4), were isolated from the roots of Iris bungei. The structures of the new compounds were established on the basis of spectroscopic methods.


