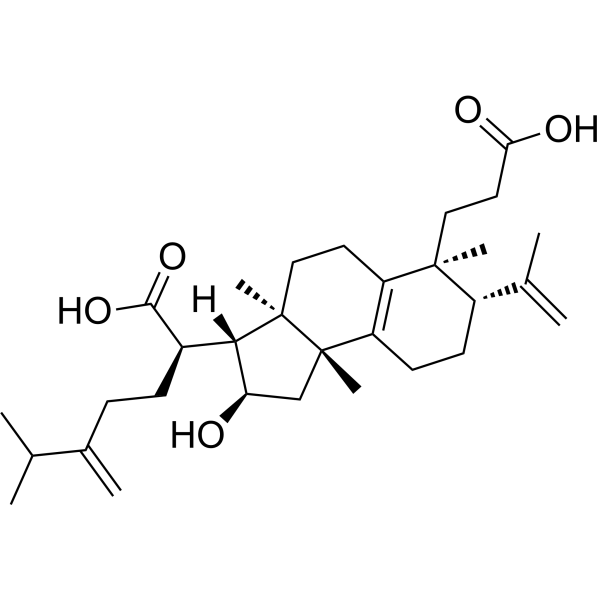
Poricoic acid HCAS# 415724-85-5 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 415724-85-5 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C31H48O5 | M.Wt | 500.71 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Poricoic acid H Dilution Calculator

Poricoic acid H Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9972 mL | 9.9858 mL | 19.9716 mL | 39.9433 mL | 49.9291 mL |
| 5 mM | 0.3994 mL | 1.9972 mL | 3.9943 mL | 7.9887 mL | 9.9858 mL |
| 10 mM | 0.1997 mL | 0.9986 mL | 1.9972 mL | 3.9943 mL | 4.9929 mL |
| 50 mM | 0.0399 mL | 0.1997 mL | 0.3994 mL | 0.7989 mL | 0.9986 mL |
| 100 mM | 0.02 mL | 0.0999 mL | 0.1997 mL | 0.3994 mL | 0.4993 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Donasine
Catalog No.:BCX1766
CAS No.:1017237-81-8
- Polyfuroside
Catalog No.:BCX1765
CAS No.:108886-03-9
- 2,4-Dihydroxy-allylbenzene-2-O-β-D-glucopyranoside
Catalog No.:BCX1764
CAS No.:1416564-61-8
- 2-Methoxy-6-pentadecyl-1,4-benzoquinone
Catalog No.:BCX1763
CAS No.:144078-11-5
- Irisquinone
Catalog No.:BCX1762
CAS No.:56495-82-0
- Inflexuside B
Catalog No.:BCX1761
CAS No.:1395048-86-8
- Quercetin-3-O-(2''-O-galloyl)-β-D-glucopyranoside
Catalog No.:BCX1760
CAS No.:69624-79-9
- Salviandulin E
Catalog No.:BCX1759
CAS No.:158994-32-2
- Glaucocalyxin D
Catalog No.:BCX1758
CAS No.:140671-02-9
- Bulnesol
Catalog No.:BCX1757
CAS No.:22451-73-6
- Inflexuside A
Catalog No.:BCX1756
CAS No.:1395048-85-7
- Cnidioside B
Catalog No.:BCX1755
CAS No.:141896-54-0
- Dugesin C
Catalog No.:BCX1768
CAS No.:1403505-97-4
- Salvileucantholide
Catalog No.:BCX1769
CAS No.:158994-33-3
- (7R,8S,8′R)-4,7-Dihydroxy-3,3′,4′-trimethoxyl-9-oxo dibenzylbutyrolactone lignan-4-O-β-D-glucopyrano...
Catalog No.:BCX1770
CAS No.:1807808-90-7
- Quercetin 3-O-β-D-xylofuranosyl-(1→2)-β-D-galactopyranoside
Catalog No.:BCX1771
CAS No.:1181635-91-5
- Dugesin B
Catalog No.:BCX1772
CAS No.:760973-66-8
- 6,7-Dihydrosalviandulin E
Catalog No.:BCX1773
CAS No.:1983982-37-1
- (±)-7-epi-Kadsurenone
Catalog No.:BCX1774
CAS No.:245648-20-8
- Arundanine
Catalog No.:BCX1775
CAS No.:618852-71-4
- Salvileucalin A
Catalog No.:BCX1776
CAS No.:1053241-84-1
- Hancinone D
Catalog No.:BCX1777
CAS No.:104973-90-2
- 3-Hydroxyirisquinone
Catalog No.:BCX1778
CAS No.:100205-29-6
- Quercetin 3-O-(6′′-caffeoyl)-β-D-galactopyranoside
Catalog No.:BCX1779
CAS No.:316354-12-8
The Cactus (Opuntia ficus-indica) Cladodes and Callus Extracts: A Study Combined with LC-MS Metabolic Profiling, In-Silico, and In-Vitro Analyses.[Pubmed:37507869]
Antioxidants (Basel). 2023 Jun 23;12(7):1329.
Opuntia ficus-indica (OF) phytochemicals have received considerable attention because of their health benefits. However, the structure-activity relationship between saponin and flavonoid antioxidant compounds among secondary metabolites has rarely been reported. In a molecular docking study, selected compounds from both Opuntia ficus-indica callus (OFC) and OF ethanol extract were found to be involved in Toll-like receptor 4 and mitogen-activated protein kinase (MAPK) signaling pathways. High affinity was specific for MAPK, and it was proposed to inhibit the oxidative and inflammatory responses with Poricoic acid H (-8.3 Kcal/mol) and rutin (-9.0 Kcal/mol). The pro-inflammatory cytokine factors at a concentration of 200 mug/mL were LPS-stimulated TNF-alpha (OFC 72.33 ng/mL, OF 66.78 ng/mL) and IL-1beta (OFC 49.10 pg/mL, OF 34.45 pg/mL), both of which significantly decreased OF (p < 0.01, p < 0.001). Taken together, increased NO, PGE(2), and pro-inflammatory cytokines were significantly decreased in a dose-dependent manner in cells pretreated with OFC and the OF extract (p < 0.05). These findings suggest that OFC and OF have important potential as natural antioxidant, anti-inflammatory agents in health-promoting foods and medicine.
Inhibition of tumor-promoting effects by poricoic acids G and H and other lanostane-type triterpenes and cytotoxic activity of poricoic acids A and G from Poria cocos.[Pubmed:11975480]
J Nat Prod. 2002 Apr;65(4):462-5.
The structures of two novel 3,4-seco-lanostane-type triterpenes isolated from the sclerotium of Poria cocos were established to be 16alpha-hydroxy-3,4-seco-lanosta-4(28),8,24-triene-3,21-dioic acid (1; poricoic acid G) and 16alpha-hydroxy-3,4-seco-24-methyllanosta-4(28),8,24(24(1))-triene-3,21-dioic acid (2; Poricoic acid H) on the basis of spectroscopic methods. These two, and eight other known compounds isolated from the sclerotium, poricoic acid B (3), poricoic acid A (4), tumulosic acid (5), dehydrotumulosic acid (6), 3-epidehydrotumulosic acid (7), polyporenic acid C (8), 25-hydroxy-3-epidehydrotumulosic acid (9), and dehydroabietic acid methyl ester (10), showed potent inhibitory effects on Epstein-Barr virus early antigen (EBV-EA) activation induced by the tumor promoter 12-O-tetradecanoylphorbol-13-acetate (TPA). Evaluation of the cytotoxicity of compounds 1 and 4 against human cancer cell lines revealed that 1 was significantly cytotoxic to leukemia HL-60 cells [GI(50) (concentration that yields 50% growth) value 39.3 nM], although it showed only moderate cytotoxicity to the other cells. Compound 4 exhibited moderate cytotoxicity to all of the cancer cell lines tested.


