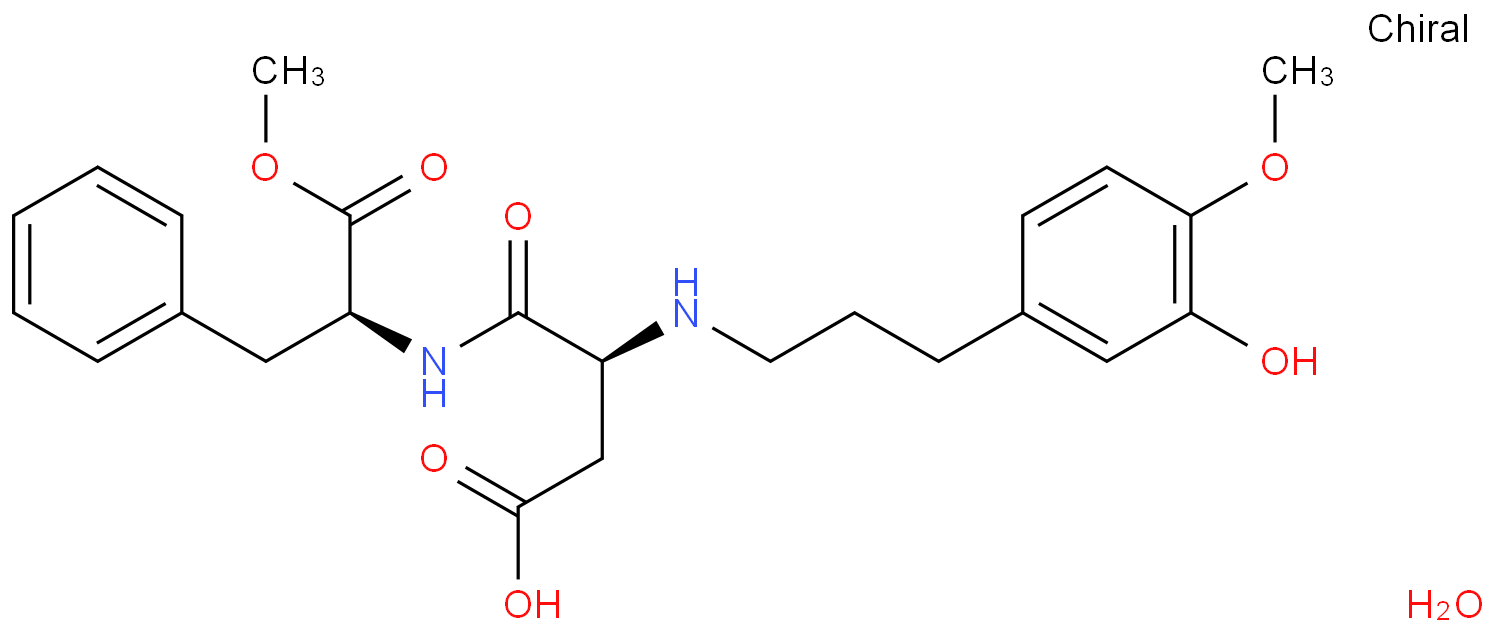
AdvantameCAS# 714229-20-6 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 714229-20-6 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C24N2O8H32 | M.Wt | 476.52 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | N-[N-[3-(3-Hydroxy-4-methoxyphenyl)propyl]-α-L-aspartyl]-L-phenylalanine 1-methyl ester monohydrate | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Advantame Dilution Calculator

Advantame Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0985 mL | 10.4927 mL | 20.9855 mL | 41.971 mL | 52.4637 mL |
| 5 mM | 0.4197 mL | 2.0985 mL | 4.1971 mL | 8.3942 mL | 10.4927 mL |
| 10 mM | 0.2099 mL | 1.0493 mL | 2.0985 mL | 4.1971 mL | 5.2464 mL |
| 50 mM | 0.042 mL | 0.2099 mL | 0.4197 mL | 0.8394 mL | 1.0493 mL |
| 100 mM | 0.021 mL | 0.1049 mL | 0.2099 mL | 0.4197 mL | 0.5246 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Wilforlide B
Catalog No.:BCX1044
CAS No.:84104-70-1
- 13β,21-Dihydroxyeurycomanone
Catalog No.:BCX1043
CAS No.:138874-44-9
- L-1,2,3,4-Tetrahydronorharman-3-carboxylic acid
Catalog No.:BCX1042
CAS No.:42438-90-4
- (-)-cis-Khellactone
Catalog No.:BCX1041
CAS No.:54712-23-1
- (+)-trans-Khellactone
Catalog No.:BCX1040
CAS No.:20516-17-0
- Regaloside H
Catalog No.:BCX1039
CAS No.:126239-77-8
- 3β,13-dihydroxy-ursan-28-oic acid-13-lactone
Catalog No.:BCX1038
CAS No.:29428-70-4
- 6-Iodo 5,7,3',4',5'-Pentamethoxyflavone
Catalog No.:BCX1037
CAS No.:1192850-63-7
- α-Norbixin
Catalog No.:BCX1036
CAS No.:626-76-6
- Isoferulic Acid
Catalog No.:BCX1035
CAS No.:537-73-5
- Acetylalkannin
Catalog No.:BCX1034
CAS No.:34232-27-4
- Agrimoniin
Catalog No.:BCX1033
CAS No.:82203-01-8
- Rhododenol
Catalog No.:BCX1046
CAS No.:69617-84-1
- Fischeroside C
Catalog No.:BCX1047
CAS No.:1307257-09-5
- 2"-Acetylhyperin
Catalog No.:BCX1048
CAS No.:439266-62-3
- Pterostilbene glucoside
Catalog No.:BCX1049
CAS No.:38967-99-6
- 3'-O-Acetylhamaudol
Catalog No.:BCX1050
CAS No.:30358-88-4
- Phenaxolactone 1
Catalog No.:BCX1051
CAS No.:147022-96-6
- 11-Methoxyyangonin
Catalog No.:BCX1052
CAS No.:2743-14-8
- Isotheaflavin
Catalog No.:BCX1053
CAS No.:31701-93-6
- Paeonidanin
Catalog No.:BCX1054
CAS No.:209969-75-5
- Isohanalpinone
Catalog No.:BCX1055
CAS No.:103476-95-5
- 3-Fucosyllactose
Catalog No.:BCX1056
CAS No.:41312-47-4
- 5-Hydroxyferulic acid
Catalog No.:BCX1057
CAS No.:1782-55-4
Simultaneous determination of nine high-intensity sweeteners in liquid and powder tabletop sweeteners.[Pubmed:37695976]
Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2023 Oct;40(10):1298-1306.
Government regulatory actions and public policies have been recently implemented in Brazil due to the excessive consumption of sugar. Therefore, it becomes relevant to determine the levels of high-intensity sweeteners in tabletop sweeteners consumed by the Brazilian population. Thus, an analytical method was developed and validated for the simultaneous determination of nine sweeteners (acesulfame potassium, aspartame, Advantame, sodium cyclamate, neotame, saccharin, sucralose, stevioside, and rebaudioside A) by using ultra-high performance liquid chromatography coupled to mass spectrometry in tandem. The sample preparation encompassed only dilution steps. The method was validated taking into account the parameters of linearity, precision, accuracy, and matrix effects. The analytes were determined in two different batches of 21 commercial liquid and powder tabletop sweeteners available on the local market, totaling 42 samples. A minimum of one and a maximum of four sweeteners were found in the analyzed products and sweeteners that were not described on the label were not detected. It is expected that the established method can be used in monitoring programs and that the presented results can contribute to exposure assessments performed nationally.
Non-sugar sweeteners and cancer: Toxicological and epidemiological evidence.[Pubmed:36870410]
Regul Toxicol Pharmacol. 2023 Mar;139:105369.
Several toxicological and epidemiological studies were published during the last five decades on non-sugar sweeteners (NSS) and cancer. Despite the large amount of research, the issue still continues to be of interest. In this review, we provided a comprehensive quantitative review of the toxicological and epidemiological evidence on the possible relation between NSS and cancer. The toxicological section includes the evaluation of genotoxicity and carcinogenicity data for acesulfame K, Advantame, aspartame, cyclamates, saccharin, steviol glycosides and sucralose. The epidemiological section includes the results of a systematic search of cohort and case-control studies. The majority of the 22 cohort studies and 46 case-control studies showed no associations. Some risks for bladder, pancreas and hematopoietic cancers found in a few studies were not confirmed in other studies. Based on the review of both the experimental data on genotoxicity or carcinogenicity of the specific NSS evaluated, and the epidemiological studies it can be concluded that there is no evidence of cancer risk associated to NSS consumption.
An updated multifaceted overview of sweet proteins and dipeptides as sugar substitutes; the chemistry, health benefits, gut interactions, and safety.[Pubmed:36461268]
Food Res Int. 2022 Dec;162(Pt A):111853.
Artificial sweeteners have become increasingly popular worldwide owing to their lower calorie content in addition to the claims of health benefits such as weight control, blood glucose level regulation in diabetics, and protection against dental caries. Nevertheless, there is still controversy regarding their safety, especially when administered over the long term, taking into account that most of the safety studies are based on animal models and only a few human studies. This review focuses on low-calorie protein/peptide sweeteners. These include artificial sweeteners, i.e. aspartame, Advantame, neotame, and alitame which are synthetic, versus those of natural origin such as thaumatin, monellin, brazzein, pentadin, mabinlin, curculin, and egg white lysozyme. We conducted a systematic literature survey to ensure the accuracy of the data regarding the chemical properties, synthesis, and industrial applications. The health benefits and safety of these sweeteners in humans are presented for the first time in context to their metabolic profiles and gut interaction.
A comprehensive database of declared high-intensity sweeteners in Brazilian commercial products and updated exposure assessment.[Pubmed:36192918]
Food Res Int. 2022 Nov;161:111899.
Government regulatory actions and public policies to reduce sugar consumption were recently implemented in Brazil. To evaluate their potential impact on the supply of products containing high-intensity sweeteners (HIS) and on dietary exposure to these substances, this study aimed to create a comprehensive database on HIS declared in Brazilian commercial products and estimate their intake through consumption of these products. The occurrence of HIS was evaluated through labeling information of 1869 commercial products available in the Brazilian market, collected between January 2021 and August 2021, and the daily intake was estimated for eight HIS (acesulfame K, Advantame, aspartame, cyclamate, steviol glycosides, neotame, saccharin and sucralose) using a deterministic approach by multiplying the maximum permitted levels of HIS in foods and beverages by the consumption data of these products. The consumption data were obtained from the report of Household Budget Survey (POF/IBGE), conducted from 2017 to 2018 through a 24-hour dietary recall applied to 46,164 individuals aged 10 years and over, which included only average data (i.e. average consumption for the general population or subgroups). The most frequent HIS in the investigated products were sucralose (26.8 %; n = 938) and acesulfame K (21.7 %; n = 759), and although the combination of sweeteners is a common practice in the food industry, there was a predominance of only one substance in the investigated products (46.7 %; n = 873). The estimated intake of HIS for average consumers was below the Acceptable Daily Intake (ADI) and does not suggest a toxicological concern. A similar scenario was observed for high consumers, except for cyclamate and steviol glycosides, which corresponded to 144 % and 131 % of their respective ADIs in the general population. To our knowledge, this is the most comprehensive database on HIS in Brazil and the most recent exposure assessment performed nationally.
The Effect of Artificial Sweeteners Use on Sweet Taste Perception and Weight Loss Efficacy: A Review.[Pubmed:35334918]
Nutrients. 2022 Mar 16;14(6):1261.
Excessive consumption of sugar-rich foods is currently one of the most important factors that has led to the development of the global pandemic of obesity. On the other hand, there is evidence that obesity contributes to reduced sensitivity to sweet taste and hormonal changes affecting appetite, leading to an increased craving for sweets. A high intake of sugars increases the caloric value of the diet and, consequently, leads to weight gain. Moreover, attention is drawn to the concept of the addictive properties of sugar and sugary foods. A potential method to reduce the energy value of diet while maintaining the sweet taste is using non-nutritive sweeteners (NNS). NNS are commonly used as table sugar substitutes. This wide group of chemical compounds features high sweetness almost without calories due to its high sweetening strength. NNS include aspartame, acesulfame-K, sucralose, saccharin, cyclamate, neohesperidin dihydrochalcone (neohesperidin DC), neotame, taumatin, and Advantame. The available evidence suggests that replacing sugar with NNS may support weight control. However, the effect of NNS on the regulation of appetite and sweet taste perception is not clear. Therefore, the review aimed to summarize the current knowledge about the use of NNS as a potential strategy for weight loss and their impact on sweet taste perception. Most studies have demonstrated that consumption of NNS-sweetened foods does not increase sweetness preference orenergy intake. Nonetheless, further research is required to determine the long-term effects of NNS on weight management.
Investigating mechanism of sweetness intensity differences through dynamic analysis of sweetener-T1R2-membrane systems.[Pubmed:34915374]
Food Chem. 2022 Apr 16;374:131807.
Knowing the mechanism of action of sweet taste receptors is important for the design of new, healthy sweeteners. However, little is known about the structures and recognition mechanisms of these receptors. 28 sweeteners were assessed by molecular docking, and 8 typical sweeteners were chosen to construct sweetener-T1R2-membrane systems to analyze interactions between receptor and sweeteners. Natural sweeteners with low-intensity sweetness, such as fructose and xylitol, were released from the Venus flytrap domain at approximately 30 ns, with displacements greater than 50 A. In contrast, artificial neotame and Advantame bound stably to the receptor, within 5 A. Van der Waals interactions were significant in high-intensity sweetener systems, imparting an energy difference of over 15 kcal/mol between neotame (artificial sweetener) and fructose (natural). These results provide a deeper understanding of the mechanisms of sweetener function and offer a new direction for the design of sweeteners.
The sensory properties and metabolic impact of natural and synthetic sweeteners.[Pubmed:33580569]
Compr Rev Food Sci Food Saf. 2021 Mar;20(2):1554-1583.
The global rise in obesity, type II diabetes, and other metabolic disorders in recent years has been attributed in part to the overconsumption of added sugars. Sugar reduction strategies often rely on synthetic and naturally occurring sweetening compounds to achieve their goals, with popular synthetic sweeteners including saccharin, cyclamate, acesulfame potassium, aspartame, sucralose, neotame, alitame, and Advantame. Natural sweeteners can be further partitioned into nutritive, including polyols, rare sugars, honey, maple syrup, and agave, and nonnutritive, which include steviol glycosides and rebaudiosides, luo han guo (monk fruit), and thaumatin. We choose the foods we consume largely on their sensory properties, an area in which these sugar substitutes often fall short. Here, we discuss the most popular synthetic and natural sweeteners, with the goal of providing an understanding of differences in the sensory profiles of these sweeteners versus sucrose, that they are designed to replace, essential for the effectiveness of sugar reduction strategies. In addition, we break down the influence of these sweeteners on metabolism, and present results from a large survey of consumers' opinions on these sweeteners. Consumer interest in clean label foods has driven a move toward natural sweeteners; however, neither natural nor synthetic sweeteners are metabolically inert. Identifying sugar replacements that not only closely imitate the sensory profile of sucrose but also exert advantageous effects on body weight and metabolism is critical in successfully the ultimate goals of reducing added sugar in the average consumer's diet. With so many options for sucrose replacement available, consumer opinion and cost, which vary widely with suagr replacements, will also play a vital role in which sweeteners are successful in widespread adoption.
Purification, characterization, and identification of 3-hydroxy-4-methoxy benzal acrolein-an intermediate of synthesizing advantame.[Pubmed:32148784]
Food Sci Nutr. 2020 Jan 1;8(2):744-753.
Advantame is a novel sweetener, and 3-hydroxy-4-methoxy benzal acrolein is an important intermediate to synthesize the sweetener. The aim of this study was to evaluate a new low-cost method to purify 3-hydroxy-4-methoxy benzal acrolein, and the crude intermediate was used as raw material. The intermediate was purified using a low-pressure column chromatography with a C(18) column, using a methanol-water (6:4, v/v) at a flow rate of 6.0 ml/min, and the loading amount of the crude intermediate in solution was 10.0 mg in total. A method for the analysis of 3-hydroxy-4-methoxy benzal acrolein was established using high-performance liquid chromatography (HPLC). This method was validated in terms of its linearity, repeatability, accuracy, detection limit, and quantitation limit. The calibration curves of 3-hydroxy-4-methoxy benzal acrolein were linear (r > .999) over a wide concentration range of 0.005-0.08 mg/ml, by comparing the ratio of signal to noise, and the detection limit was 5.0 ng/ml and the quantification limit was 15.0 ng/ml. Good repeatability was obtained (RSD < 2%, n = 6), and the recoveries calculated using mixed sample previously quantified ranged from 94.5% to 106.37%. As long as, this method has been successfully applied to the analysis of 3-hydroxy-4-methoxy benzal acrolein; therefore, the method can be put into practical use during the industrial synthesis and real-time detection of the intermediate.
Engineered C-N Lyase: Enantioselective Synthesis of Chiral Synthons for Artificial Dipeptide Sweeteners.[Pubmed:31625664]
Angew Chem Int Ed Engl. 2020 Jan 2;59(1):429-435.
Aspartic acid derivatives with branched N-alkyl or N-arylalkyl substituents are valuable precursors to artificial dipeptide sweeteners such as neotame and Advantame. The development of a biocatalyst to synthesize these compounds in a single asymmetric step is an as yet unmet challenge. Reported here is an enantioselective biocatalytic synthesis of various difficult N-substituted aspartic acids, including N-(3,3-dimethylbutyl)-l-aspartic acid and N-[3-(3-hydroxy-4-methoxyphenyl)propyl]-l-aspartic acid, precursors to neotame and Advantame, respectively, using an engineered variant of ethylenediamine-N,N'-disuccinic acid (EDDS) lyase from Chelativorans sp. BNC1. This engineered C-N lyase (mutant D290M/Y320M) displayed a remarkable 1140-fold increase in activity for the selective hydroamination of fumarate compared to that of the wild-type enzyme. These results present new opportunities to develop practical multienzymatic processes for the more sustainable and step-economic synthesis of an important class of food additives.
Effects of Sweeteners on the Gut Microbiota: A Review of Experimental Studies and Clinical Trials.[Pubmed:30721958]
Adv Nutr. 2019 Jan 1;10(suppl_1):S31-S48.
The consumption of sugar-free foods is growing because of their low-calorie content and the health concerns about products with high sugar content. Sweeteners that are frequently several hundred thousand times sweeter than sucrose are being consumed as sugar substitutes. Although nonnutritive sweeteners (NNSs) are considered safe and well tolerated, their effects on glucose intolerance, the activation of sweet taste receptors, and alterations to the composition of the intestinal microbiota are controversial. This review critically discusses the evidence supporting the effects of NNSs, both synthetic sweeteners (acesulfame K, aspartame, cyclamate, saccharin, neotame, Advantame, and sucralose) and natural sweeteners (NSs; thaumatin, steviol glucosides, monellin, neohesperidin dihydrochalcone, and glycyrrhizin) and nutritive sweeteners (polyols or sugar alcohols) on the composition of microbiota in the human gut. So far, only saccharin and sucralose (NNSs) and stevia (NS) change the composition of the gut microbiota. By definition, a prebiotic is a nondigestible food ingredient, but some polyols can be absorbed, at least partially, in the small intestine by passive diffusion: however, a number of them, such as isomaltose, maltitol, lactitol, and xylitol, can reach the large bowel and increase the numbers of bifidobacteria in humans. Further research on the effects of sweeteners on the composition of the human gut microbiome is necessary.
Development of a highly sensitive liquid chromatography with tandem mass spectrometry method for the qualitative and quantitative analysis of high-intensity sweeteners in processed foods.[Pubmed:30718058]
J Chromatogr A. 2019 May 10;1592:64-70.
A new method for the simultaneous determination of two sweeteners (Advantame and Neotame) in processed foods using liquid chromatography (LC) with tandem mass spectrometry(MS/MS) was developed herein. Chromatographic separations were performed using an ACQUITY UPLC CSH C18 column at 40 degrees C via a mobile phase comprising 10-mmol/L ammonium formate and methanol. Samples were prepared via rapid dialysis using 30% methanol solution in a thermostatic shaker set at 160 rpm and 50 degrees C for 1 h. The matrix in the test solution had no effect on the identification and quantification of the compound without a clean-up step using solid-phase extraction (SPE). This method satisfied all validation criteria with a limit of quantification (LOQ) of 0.01 mug/g for all samples. Using this method, the amounts of Advantame and Neotame in 24 processed foods were subsequently investigated, with the results indicating their detection beyond the lower LOQ. Moreover, a multiple reaction monitoring information-dependent acquisition-enhanced product ion (MRM-IDA-EPI) method was developed and described to further enhance product-identification ability.
Measuring Artificial Sweeteners Toxicity Using a Bioluminescent Bacterial Panel.[Pubmed:30257473]
Molecules. 2018 Sep 25;23(10):2454.
Artificial sweeteners have become increasingly controversial due to their questionable influence on consumers' health. They are introduced in most foods and many consume this added ingredient without their knowledge. Currently, there is still no consensus regarding the health consequences of artificial sweeteners intake as they have not been fully investigated. Consumption of artificial sweeteners has been linked with adverse effects such as cancer, weight gain, metabolic disorders, type-2 diabetes and alteration of gut microbiota activity. Moreover, artificial sweeteners have been identified as emerging environmental pollutants, and can be found in receiving waters, i.e., surface waters, groundwater aquifers and drinking waters. In this study, the relative toxicity of six FDA-approved artificial sweeteners (aspartame, sucralose, saccharine, neotame, Advantame and acesulfame potassium-k (ace-k)) and that of ten sport supplements containing these artificial sweeteners, were tested using genetically modified bioluminescent bacteria from E. coli. The bioluminescent bacteria, which luminesce when they detect toxicants, act as a sensing model representative of the complex microbial system. Both induced luminescent signals and bacterial growth were measured. Toxic effects were found when the bacteria were exposed to certain concentrations of the artificial sweeteners. In the bioluminescence activity assay, two toxicity response patterns were observed, namely, the induction and inhibition of the bioluminescent signal. An inhibition response pattern may be observed in the response of sucralose in all the tested strains: TV1061 (MLIC = 1 mg/mL), DPD2544 (MLIC = 50 mg/mL) and DPD2794 (MLIC = 100 mg/mL). It is also observed in neotame in the DPD2544 (MLIC = 2 mg/mL) strain. On the other hand, the induction response pattern may be observed in its response in saccharin in TV1061 (MLIndC = 5 mg/mL) and DPD2794 (MLIndC = 5 mg/mL) strains, aspartame in DPD2794 (MLIndC = 4 mg/mL) strain, and ace-k in DPD2794 (MLIndC = 10 mg/mL) strain. The results of this study may help in understanding the relative toxicity of artificial sweeteners on E. coli, a sensing model representative of the gut bacteria. Furthermore, the tested bioluminescent bacterial panel can potentially be used for detecting artificial sweeteners in the environment, using a specific mode-of-action pattern.
[Determination of a new sweetener advantame in food by ultra-high performance liquid chromatography-tandem mass spectrometry].[Pubmed:30136544]
Se Pu. 2018 Jul 8;36(7):700-704.
A new ultra-high performance liquid chromatography-tandem mass spectrometry with triple quadrupole method was developed, and validated for screening and confirmation of the new sweetener Advantame in food. The sample was extracted with methanol-water (50:50, v/v), followed by centrifugation. The supernatant was then filtered through a membrane filter. The determinant was performed on Agilent SB-C18 (150 mmx2.1 mm, 5 mum) column with gradient elution. The target compound was carried out by positive electrospray ionization (ESI(+)) under the multiple reaction monitoring (MRM) mode. While the qualitative screening was achieved with retention time and qualitative ion, the confirmation analysis was achieved with peak area and quantitative ion. The results showed that the limit of detection (LOD, S/N >/= 3) and the limit of quantification (LOQ, S/N >/= 10)were 0.03 and 0.10 mg/kg, respectively. The average spiked recoveries showed a variation from 80.3 to 98.0% for the three fortification levels. The results showed good linear relationships in the range of 0.01-1.0 mg/L of Advantame with correlation coefficients above 0.997. This procedure was noticeably rapid, simple, sensitive, and accurate. Therefore, this method is suitable for batch determination of the new sweetener Advantame in beverages, yogurt, and jelly.
Evaluation of certain food additives and contaminants. Eightieth report of the Joint FAO/WHO Expert Committee on Food Additives.[Pubmed:27514183]
World Health Organ Tech Rep Ser. 2016;(995):1-114, back cover.
This report represents the conclusions of a Joint FAO/WHO Expert Committee convened to evaluate the safety of various food additives and contaminants and to prepare specifications for identity and purity. The first part of the report contains a brief description of general considerations addressed at the meeting, including updates on matters of interest to the work of the Committee. A summary follows of the Committee's evaluations of technical, toxicological and/or dietary exposure data for seven food additives (benzoates; lipase from Fusarium heterosporum expressed in Ogataea polymorpha; magnesium stearate; maltotetraohydrolase from Pseudomonas stutzeri expressed in Bacillus licheniformis; mixed beta-glucanase, cellulase and xylanase from Rasamsonia emersonii; mixed beta-glucanase and xylanase from Disporotrichum dimorphosporum; polyvinyl alcohol (PVA)- polyethylene glycol (PEG) graft copolymer) and two groups of contaminants (non-dioxin-like polychlorinated biphenyls and pyrrolizidine alkaloids). Specifications for the following food additives were revised or withdrawn: Advantame; annatto extracts (solavnt extracted bixin, ad solvent-extracted norbixin); food additives containing aluminium and/or silicon (aluminium silicate; calcium aluminium silicate; calcium silicate; silicon dioxide, amorphous; sodium aluminium silicate); and glycerol ester of gum rosin. Annexed to the report are tables or text summarizing the toxicological and dietary exposure information and information on specifications as well as the Committees recommendations on the food additives and contaminants considered at this meeting.


