AgathisflavoneCAS# 28441-98-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 28441-98-7 | SDF | File under preparation. |
| PubChem ID | 5281599 | Appearance | Yellow powder |
| Formula | C30H18O10 | M.Wt | 538.5 |
| Type of Compound | Biflavanone | Storage | Desiccate at -20°C |
| Synonyms | 6,8''-Biapigenin | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
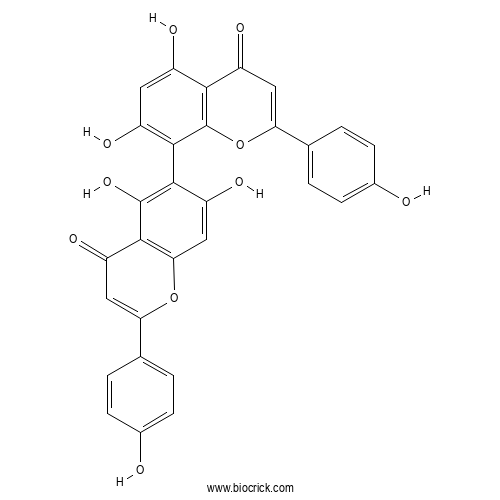
| Chemical Name | 8-[5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxochromen-6-yl]-5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one | ||
| SMILES | C1=CC(=CC=C1C2=CC(=O)C3=C(O2)C=C(C(=C3O)C4=C(C=C(C5=C4OC(=CC5=O)C6=CC=C(C=C6)O)O)O)O)O | ||
| Standard InChIKey | BACLASYRJRZXMY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C30H18O10/c31-15-5-1-13(2-6-15)22-11-20(36)26-24(39-22)12-21(37)27(29(26)38)28-18(34)9-17(33)25-19(35)10-23(40-30(25)28)14-3-7-16(32)8-4-14/h1-12,31-34,37-38H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Agathisflavone Dilution Calculator

Agathisflavone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.857 mL | 9.2851 mL | 18.5701 mL | 37.1402 mL | 46.4253 mL |
| 5 mM | 0.3714 mL | 1.857 mL | 3.714 mL | 7.428 mL | 9.2851 mL |
| 10 mM | 0.1857 mL | 0.9285 mL | 1.857 mL | 3.714 mL | 4.6425 mL |
| 50 mM | 0.0371 mL | 0.1857 mL | 0.3714 mL | 0.7428 mL | 0.9285 mL |
| 100 mM | 0.0186 mL | 0.0929 mL | 0.1857 mL | 0.3714 mL | 0.4643 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Roburin E
Catalog No.:BCN0809
CAS No.:137343-79-4
- Roburin D
Catalog No.:BCN0808
CAS No.:136199-93-4
- Roburin C
Catalog No.:BCN0807
CAS No.:137490-45-0
- Roburin B
Catalog No.:BCN0806
CAS No.:137370-65-1
- Roburin A
Catalog No.:BCN0805
CAS No.:132864-75-6
- Gambogoic acid A
Catalog No.:BCN0804
CAS No.:8879-23-49-1
- Moreollic acid
Catalog No.:BCN0803
CAS No.:173792-68-2
- Epigambogellic acid
Catalog No.:BCN0802
CAS No.:1352191-85-5
- Cannabisin M
Catalog No.:BCN0801
CAS No.:1831134-13-4
- 11beta,13-Dihydrolactucin
Catalog No.:BCN0800
CAS No.:83117-63-9
- 2-Phenylethyl-beta-glucopyranoside
Catalog No.:BCN0799
CAS No.:18997-54-1
- Naringenin 7-O-gentiobioside
Catalog No.:BCN0798
CAS No.:104154-33-8
- Rhusflavanone
Catalog No.:BCN0811
CAS No.:53060-72-3
- Elgonica dimer A
Catalog No.:BCN0813
CAS No.:132210-48-1
- 4-Methoxybenzoylacetic acid
Catalog No.:BCN0814
CAS No.:13422-77-0
- Cajanine
Catalog No.:BCN0815
CAS No.:87402-84-4
- Senkyunolide N
Catalog No.:BCN0816
CAS No.:140694-58-2
- Dehydrodicatechin A
Catalog No.:BCN0817
CAS No.:36048-23-4
- Barbaloin-related compound A
Catalog No.:BCN0818
CAS No.:473225-21-7
- Barbaloin-related compound B
Catalog No.:BCN0819
CAS No.:473225-22-8
- 3beta-(alpha-L-Arabinopyranosyloxy)urs-12,18-dien-28-oic acid beta-D-glucopyranosyl ester
Catalog No.:BCN0820
CAS No.:435269-07-1
- 3beta-Hydroxyurs-12,18-dien-28-oic acid beta-D-glucopyranosyl ester
Catalog No.:BCN0821
CAS No.:434942-42-4
- 10-Methoxygambogenic acid
Catalog No.:BCN0822
CAS No.:2095102-72-8
- 10-Hydroxyaloin B
Catalog No.:BCN0823
CAS No.:134863-92-6
Phenolics as GABAA Receptor Ligands: An Updated Review.[Pubmed:35335130]
Molecules. 2022 Mar 8;27(6). pii: molecules27061770.
Natural products can act as potential GABA modulators, avoiding the undesirable effects of traditional pharmacology used for the inhibition of the central nervous system such as benzodiazepines (BZD). Phenolics, especially flavonoids and phlorotannins, have been considered as modulators of the BZD-site of GABAA receptors (GABAARs), with sedative, anxiolytic or anticonvulsant effects. However, the wide chemical structural variability of flavonoids shows their potential action at more than one additional binding site on GABAARs, which may act either negatively, positively, by neutralizing GABAARs, or directly as allosteric agonists. Therefore, the aim of the present review is to compile and discuss an update of the role of phenolics, namely as pharmacological targets involving dysfunctions of the GABA system, analyzing both their different compounds and their mechanism as GABAergic modulators. We focus this review on articles written in English since the year 2010 until the present. Of course, although more research would be necessary to fully establish the type specificity of phenolics and their pharmacological activity, the evidence supports their potential as GABAAR modulators, thereby favoring their inclusion in the development of new therapeutic targets based on natural products. Specifically, the data compiled in this review allows for the directing of future research towards ortho-dihydroxy diterpene galdosol, the flavonoids isoliquiritigenin (chalcone), rhusflavone and Agathisflavone (biflavonoids), as well as the phlorotannins, dieckol and triphlorethol A. Clinically, flavonoids are the most interesting phenolics due to their potential as anticonvulsant and anxiolytic drugs, and phlorotannins are also of interest as sedative agents.
Phytochemical analysis on the leaves of Agathis microstachya J.F. Bailey & C.T. White.[Pubmed:34957868]
Nat Prod Res. 2021 Dec 27:1-5.
The first phytochemical analysis on the leaves of Agathis microstachya J.F. Bailey & C.T. White collected in Rome was reported in this work. The study evidenced the presence of four compounds i.e., 7,4'''-dimethoxy-Agathisflavone (1), 7,7''-dimethoxy-cupressuflavone (2), dactylifric acid (3) and shikimic acid (4) which were identified by means of spectroscopic techniques. Compounds (1, 2, 4) were reported in the species for the first time as well as this is the second report on the presence of dactylifric acid (3) in the whole Araucariaceae family. The absence of diterpenoids from the studied accession is also important. All these chemotaxonomic aspects were discussed.
Standardization of Juniperus macrocarpa Sibt. & Sm. and Juniperus excelsa M. Bieb. Extracts with Carbohydrate Digestive Enzyme Inhibitory and Antioxidant Activities.[Pubmed:34904000]
Iran J Pharm Res. 2021 Summer;20(3):441-455.
Juniperus species growing in Turkey are used for various medicinal purposes in traditional folk medicine. We aimed to evaluate in-vitro antidiabetic (alpha-glucosidase and alpha-amylase inhibition assays), antiobesity (pancreatic lipase inhibition assay), and antioxidant (ABTS and DPPH radical scavenging activities, ferric reducing activity, metal chelating activity, and phosphomolybdenum assay) activities of the extracts obtained from branches, leaves, and fruits of Juniperus macrocarpa and Juniperus excelsa. The branch (IC50 = 67.1 +/- 1.7 microg/mL) and leaf ethyl acetate extracts (IC50 = 83.4 +/- 0.8 microg/mL) of J. macrocarpa exhibited the strongest activity on the alpha-glucosidase enzyme. Besides that, J. excelsa leaf methanol extract exerted remarkable alpha-amylase inhibitory activity (IC50 = 950.1 +/- 3.5 microg/mL). Only J. macrocarpa branch and J. excelsa leaf ethyl acetate extract slightly inhibited pancreatic lipase enzyme with 2963.3 +/- 736.4 and 2343 +/- 557.8 microg/mL IC50 values, respectively. The RP-HPLC-DAD analysis results demonstrated that the more active J. macrocarpa extracts are richer in Agathisflavone, amentoflavone, and umbelliferone than J. excelsa extracts. With this study, it is concluded that J. macrocarpa branch and leaf ethyl acetate extracts may be a new source of alpha-glucosidase enzyme inhibitory activity and Agathisflavone, amentoflavone can be used in the standardization of the extracts.
Chemical composition and anti-Mayaro virus activity of Schinus terebinthifolius fruits.[Pubmed:34631977]
Virusdisease. 2021 Sep;32(3):526-534.
Brazilian traditional medicine has explored the antiviral properties of many plant extracts, including those from the Brazilian pepper tree, Schinus terebinthifolius. In the present study, we investigated the chemical composition and anti-mayaro virus (MAYV) activity of S. terebinthifolius fruit. Extensive virucidal activity (more than 95%) was detected for the ethyl acetate extract and the isolated biflavonoids. From the ethyl acetate extract of Schinus terebinthifolius fruits, two bioflavonoids were isolated ((2S, 2''S)-2,3,2'',3''-tetrahydroamentoflavone and Agathisflavone), which showed strong virucidal activity against Mayaro virus. Furthermore, several other compounds like terpenes and phenolics were identified by hyphenated techniques (GC-MS, LC-MS and HPLC-UV), as well as by mass spectrometry. Immunofluorescence assay confirmed antiviral activity and transmission electron microscopy revealed damage in viral particles treated with biflavonoids. The data suggest the direct action of the extract and the biflavonoids on the virus particles. The biflavonoids tetrahydroamentoflavone and Agathisflavone had strong virucidal activity and reduced MAYV infection. Supplementary Information: The online version contains supplementary material available at 10.1007/s13337-021-00698-z.
Exploring Phytochemicals of Traditional Medicinal Plants Exhibiting Inhibitory Activity Against Main Protease, Spike Glycoprotein, RNA-dependent RNA Polymerase and Non-Structural Proteins of SARS-CoV-2 Through Virtual Screening.[Pubmed:34305589]
Front Pharmacol. 2021 Jul 8;12:667704.
Severe Acute Respiratory Syndrome Corona Virus 2 (SARS-CoV-2) being a causative agent for global pandemic disease nCOVID'19, has acquired much scientific attention for the development of effective vaccines and drugs. Several attempts have been made to explore repurposing existing drugs known for their anti-viral activities, and test the traditional herbal medicines known for their health benefiting and immune-boosting activity against SARS-CoV-2. In this study, efforts were made to examine the potential of 605 phytochemicals from 37 plant species (of which 14 plants were endemic to India) and 139 antiviral molecules (Pubchem and Drug bank) in inhibiting SARS-CoV-2 multiple protein targets through a virtual screening approach. Results of our experiments revealed that SARS-CoV-2 M(Pro) shared significant disimilarities against SARS-CoV M(Pro) and MERS-CoV M(Pro) indicating the need for discovering novel drugs. This study has screened the phytochemical cyanin (Zingiber officinale) which may exhibit broad-spectrum inhibitory activity against main proteases of SARS-CoV-2, SARS-CoV and MERS-CoV with binding energies of (-) 8.3 kcal/mol (-) 8.2 kcal/mol and (-) 7.7 kcal/mol respectively. Amentoflavone, Agathisflavone, catechin-7-o-gallate and chlorogenin were shown to exhibit multi-target inhibitory activity. Further, Mangifera indica, Anacardium occidentale, Vitex negundo, Solanum nigrum, Pedalium murex, Terminalia chebula, Azadirachta indica, Cissus quadrangularis, Clerodendrum serratum and Ocimum basilicumaree reported as potential sources of phytochemicals for combating nCOVID'19. More interestingly, this study has highlighted the anti-viral properties of the traditional herbal formulation "Kabasura kudineer" recommended by AYUSH, a unit of Government of India. Short listed phytochemicals could be used as leads for future drug design and development. Genomic analysis of identified herbal plants will help in unraveling molecular complexity of therapeutic and anti-viral properties which proffer lot of chance in the pharmaceutical field for researchers to scout new drugs in drug discovery.
Agathisflavone Modifies Microglial Activation State and Myelination in Organotypic Cerebellar Slices Culture.[Pubmed:33881709]
J Neuroimmune Pharmacol. 2021 Apr 21. pii: 10.1007/s11481-021-09991-6.
Oligodendrocytes produce the myelin that is critical for rapid neuronal transmission in the central nervous system (CNS). Disruption of myelin has devastating effects on CNS function, as in the demyelinating disease multiple sclerosis (MS). Microglia are the endogenous immune cells of the CNS and play a central role in demyelination and repair. There is a need for new potential therapies that regulate myelination and microglia to promote repair. Agathisflavone (FAB) is a non-toxic flavonoid that is known for its anti-inflammatory and neuroprotective properties. Here, we examined the effects of FAB (5-50 muM) on myelination and microglia in organotypic cerebellar slices prepared from P10-P12 Sox10-EGFP and Plp1-DsRed transgenic mice. Immunofluorescence labeling for myelin basic protein (MBP) and neurofilament (NF) demonstrates that FAB significantly increased the proportion of MBP + /NF + axons but did not affect the overall number of oligodendroglia or axons, or the expression of oligodendroglial proteins CNPase and MBP. FAB is known to be a phytoestrogen, but blockade of alpha- or beta- estrogen receptors (ER) indicated the myelination promoting effects of FAB were not mediated by ER. Examination of microglial responses by Iba1 immunohistochemistry demonstrated that FAB markedly altered microglial morphology, characterized by smaller somata and reduced branching of their processes, consistent with a decreased state of activation, and increased Iba1 protein expression. The results provide evidence that FAB increases the extent of axonal coverage by MBP immunopositive oligodendroglial processes and has a modulatory effect upon microglial cells, which are important therapeutic strategies in multiple neuropathologies.
Natural biflavonoids as potential therapeutic agents against microbial diseases.[Pubmed:33493916]
Sci Total Environ. 2021 May 15;769:145168.
Microbes broadly constitute several organisms like viruses, protozoa, bacteria, and fungi present in our biosphere. Fast-paced environmental changes have influenced contact of human populations with newly identified microbes resulting in diseases that can spread quickly. These microbes can cause infections like HIV, SARS-CoV2, malaria, nosocomial Escherichia coli, methicillin-resistant Staphylococcus aureus (MRSA), or Candida infection for which there are no available vaccines/drugs or are less efficient to prevent or treat these infections. In the pursuit to find potential safe agents for therapy of microbial infections, natural biflavonoids like amentoflavone, tetrahydroamentoflavone, ginkgetin, bilobetin, morelloflavone, Agathisflavone, hinokiflavone, Garcinia biflavones 1 (GB1), Garcinia biflavones 2 (GB2), robustaflavone, strychnobiflavone, ochnaflavone, dulcisbiflavonoid C, tetramethoxy-6,6''-bigenkwanin and other derivatives isolated from several species of plants can provide effective starting points and become a source of future drugs. These biflavonoids show activity against influenza, severe acute respiratory syndrome (SARS), dengue, HIV-AIDS, coxsackieviral, hepatitis, HSV, Epstein-Barr virus (EBV), protozoal (Leishmaniasis, Malaria) infections, bacterial and fungal infections. Some of the biflavonoids can provide antiviral and protozoal activity by inhibition of neuraminidase, chymotrypsin-like protease, DV-NS5 RNA dependant RNA polymerase, reverse transcriptase (RT), fatty acid synthase, DNA polymerase, UL54 gene expression, Epstein-Barr virus early antigen activation, recombinant cysteine protease type 2.8 (r-CPB2.8), Plasmodium falciparum enoyl-acyl carrier protein (ACP) reductase or cause depolarization of parasitic mitochondrial membranes. They may also provide anti-inflammatory therapeutic activity against the infection-induced cytokine storm. Considering the varied bioactivity of these biflavonoids against these organisms, their structure-activity relationships are derived and wherever possible compared with monoflavones. Overall, this review aims to highlight these natural biflavonoids and briefly discuss their sources, reported mechanism of action, pharmacological uses, and comment on resistance mechanism, flavopiridol repurposing and the bioavailability aspects to provide a starting point for anti-microbial research in this area.
Reverted effect of mesenchymal stem cells in glioblastoma treated with agathisflavone and its selective antitumoral effect on cell viability, migration, and differentiation via STAT3.[Pubmed:33368262]
J Cell Physiol. 2021 Jul;236(7):5022-5035.
Glioblastoma is the most lethal tumor of the central nervous system, presenting a very poor prognostic, with a survival around 16 months. The interaction of mesenchymal stem cells and tumor cells has been studied, showing a bias in their role favoring or going against aggressiveness. Natural products such as flavonoids have showed their anticancer properties and the synergic potential with the activation of microenvironment cells to inhibit tumor progression. Agathisflavone is a flavonoid studied in neurodegenerative diseases and cancer. The present study investigated the effect of flavonoid in the viability of heterogeneous glioblastoma (GBM) cells considering a coculture or conditioned medium of mesenchymal stem cells (MSCs) effect, as well as the dose-dependent effect of this flavonoid in tumor migration and differentiation via STAT3. Agathisflavone (3-10 muM) induced dose-dependent toxicity to GL-15 and U373 human GBM cells, since 24 h after treatments. It was not toxic to human MSC but modified the pattern of interaction with GBM cells. Agathisflavone also inhibited migration and increased differentiation of human GBM cells, associated with the reduction on the expression of STAT3. These results demonstrate that the flavonoid Agathisflavone had a direct anti-glioma effect. However, could be observed its effect in MSCs response that may have an impact in controlling GBM growth and aggressiveness, an important factor to consider for new therapies.
Biflavonoids from Rhus succedanea as probable natural inhibitors against SARS-CoV-2: a molecular docking and molecular dynamics approach.[Pubmed:33300454]
J Biomol Struct Dyn. 2020 Dec 10:1-13.
The recent outbreak of SARS-CoV-2 has quickly become a worldwide pandemic and generated panic threats for both the human population and the global economy. The unavailability of effective vaccines or drugs has enforced researchers to hunt for a potential drug to combat this virus. Plant-derived phytocompounds are of applicable interest in the search for novel drugs. Bioflavonoids from Rhus succedanea are already reported to exert antiviral activity against RNA viruses. SARS-CoV-2 Mpro protease plays a vital role in viral replication and therefore can be considered as a promising target for drug development. A computational approach has been employed to search for promising potent bioflavonoids from Rhus succedanea against SARS-CoV-2 Mpro protease. Binding affinities and binding modes between the biflavonoids and Mpro enzyme suggest that all six biflavonoids exhibit possible interaction with the Mpro catalytic site (-19.47 to -27.04 kcal/mol). However, Amentoflavone (-27.04 kcal/mol) and Agathisflavone (-25.87 kcal/mol) interact strongly with the catalytic residues. Molecular dynamic simulations (100 ns) further revealed that these two biflavonoids complexes with the Mpro enzyme are highly stable and are of less conformational fluctuations. Also, the hydrophobic and hydrophilic surface mapping on the Mpro structure as well as biflavonoids were utilized for the further lead optimization process. Altogether, our findings showed that these natural biflavonoids can be utilized as promising SARS-CoV-2 Mpro inhibitors and thus, the computational approach provides an initial footstep towards experimental studies in in vitro and in vivo, which is necessary for the therapeutic development of novel and safe drugs to control SARS-CoV-2. Communicated by Ramaswamy H. SarmaResearch highlightsRhus succedanea biflavonoids have antiviral activity.The molecular interactions and molecular dynamics displayed that all six biflavonoids bound with a good affinity to the same catalytic site of Mpro.The compound Amentoflavone has a strong binding affinity (-27.0441 kcal/mol) towards Mpro.The binding site properties of SARS-CoV-2-Mpro can be utilized in a novel discovery and lead optimization of the SARS-CoV-2-Mpro inhibitor.
Computational selection of flavonoid compounds as inhibitors against SARS-CoV-2 main protease, RNA-dependent RNA polymerase and spike proteins: A molecular docking study.[Pubmed:33110386]
Saudi J Biol Sci. 2021 Jan;28(1):448-458.
An outbreak of Coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 has been recognized as a global health concern. Since, no specific antiviral drug is proven effective for treatment against COVID-19, identification of new therapeutics is an urgent need. In this study, flavonoid compounds were analyzed for its inhibitory potential against important protein targets of SARS-CoV-2 using computational approaches. Virtual docking was performed for screening of flavonoid compounds retrieved from PubChem against the main protease of SARS-CoV-2 using COVID-19 docking server. The cut off of dock score was set to >-9 kcal/mol and screened compounds were individually docked against main protease, RNA-dependent RNA polymerase, and spike proteins using AutoDock 4.1 software. Finally, lead flavonoid compounds were subjected to ADMET analysis. A total of 458 flavonoid compounds were virtually screened against main protease target and 36 compounds were selected based on the interaction energy value >-9 kcal/mol. Furthermore, these compounds were individually docked against protein targets and top 10 lead compounds were identified. Among the lead compounds, Agathisflavone showed highest binding energy value of -8.4 kcal/mol against main protease, Albireodelphin showed highest dock score of -9.8 kcal/mol and -11.2 kcal/mol against RdRp, and spike proteins, respectively. Based on the high dock score and ADMET properties, top 5 lead molecules such as Albireodelphin, Apigenin 7-(6''-malonylglucoside), Cyanidin-3-(p-coumaroyl)-rutinoside-5-glucoside, Delphinidin 3-O-beta-D-glucoside 5-O-(6-coumaroyl-beta-D-glucoside) and (-)-Maackiain-3-O-glucosyl-6''-O-malonate were identified as potent inhibitors against main protease, RdRp, and spike protein targets of SARS-CoV-2. These all compounds are having non-carcinogenic and non-mutagenic properties. This study finding suggests that the screened compounds include Albireodelphin, Apigenin 7-(6''-malonylglucoside), Cyanidin-3-(p-coumaroyl)-rutinoside-5-glucoside, Delphinidin 3-O-beta-D-glucoside 5-O-(6-coumaroyl-beta-D-glucoside) and (-)-Maackiain-3-O-glucosyl-6''-O-malonate could be the potent inhibitors of SARS-CoV-2 targets.
Activity-guided isolation of alpha-amylase, alpha-glucosidase, and pancreatic lipase inhibitory compounds from Rhus coriaria L.[Pubmed:32895959]
J Food Sci. 2020 Oct;85(10):3220-3228.
The leaves and fruits of Rhus coriaria are traditionally used in Turkey for the treatment of diabetes. The aim of the present study is to determine alpha-amylase, alpha-glucosidase, and pancreatic lipase inhibitory activities of R. coriaria leaf and fruit ethanol extracts (80%), and to isolate active compounds against these enzymes. As a result of the activity-guided isolation, the active compounds were determined as the amentoflavone, Agathisflavone, and 1,2,3,4,6-penta-O-galloyl-beta-glucopyranose. Agathisflavone, amentoflavone, and penta-O-galloyl-beta-glucopyranose inhibited alpha-glucosidase with 11.4 +/- 0.9, 11.3 +/- 0.7, and 4.1 +/- 0.1 microM IC50 values, respectively. Furthermore, penta-O-galloyl-beta-glucopyranose inhibited alpha-amylase with 6.32 +/- 0.18 microM IC50 . These three compounds also significantly inhibited (P < 0.05) pancreatic lipase. The results of high-performance liquid chromatography analysis showed that penta-O-galloyl-beta-D-glycopyranose was one of the main compounds in both fruit and leaf extracts. Therefore, it may be considered that R. coriaria fruit and leaf extracts can be standardized on this substance and used in the development of both medicinal products and functional food for diabetes. PRACTICAL APPLICATION: Rhus coriaria (Sumac) is one of the plants that is well known and used around the world as a spice. It is also used against diabetes traditionally. The determination of effective compounds can lead to the standardization and development of both medicinal products and functional foods for diabetes. While the fruits of the plant are used as a spice all around the world, the leaves are generally throw away; therefore, the usage of the leaves to the food and medical industry can lead to beneficial effects on the economy.
The Flavonoid Agathisflavone from Poincianella pyramidalis Prevents Aminochrome Neurotoxicity.[Pubmed:32588357]
Neurotox Res. 2020 Oct;38(3):579-584.
Flavonoids have been suggested to protect dopaminergic neurons in Parkinson's disease based on studies that used exogenous neurotoxins. In this study, we tested the protective ability of Agathisflavone in SH-SY5Y cells exposed to the endogenous neurotoxin aminochrome. The ability of aminochrome to induce loss of lysosome acidity is an important mechanism of its neurotoxicity. We demonstrated that the flavonoid inhibited cellular death and lysosomal dysfunction induced by aminochrome. In addition, we demonstrated that the protective effect of Agathisflavone was suppressed by antagonists of estrogen receptors (ERalpha and ERbeta). These results suggest lysosomal protection and estrogen signaling as mechanisms involved in Agathisflavone neuroprotection in a Parkinson's disease study model.
The flavonoid agathisflavone modulates the microglial neuroinflammatory response and enhances remyelination.[Pubmed:32534098]
Pharmacol Res. 2020 Sep;159:104997.
Myelin loss is the hallmark of the demyelinating disease multiple sclerosis (MS) and plays a significant role in multiple neurodegenerative diseases. A common factor in all neuropathologies is the central role of microglia, the intrinsic immune cells of the central nervous system (CNS). Microglia are activated in pathology and can have both pro- and anti-inflammatory functions. Here, we examined the effects of the flavonoid Agathisflavone on microglia and remyelination in the cerebellar slice model following lysolecithin induced demyelination. Notably, Agathisflavone enhances remyelination and alters microglial activation state, as determined by their morphology and cytokine profile. Furthermore, these effects of Agathisflavone on remyelination and microglial activation were inhibited by blockade of estrogen receptor alpha. Thus, our results identify Agathisflavone as a novel compound that may act via ER to regulate microglial activation and enhance remyelination and repair.
Effects of structural differences on the antibacterial activity of biflavonoids from fruits of the Brazilian peppertree (Schinus terebinthifolius Raddi).[Pubmed:32466911]
Food Res Int. 2020 Jul;133:109134.
Flavonoids, synthesized by plants across all families and therefore found in a huge variety, possess a diverse range of pharmacological properties. Direct antibacterial and synergistic activities as well as the inhibition of several bacterial virulence factors are known. Besides the mode of action, it is important to understand the structure-activity relationship to identify key structural characteristics. This study aimed to identify biflavonoids with antibacterial activity from Schinus terebinthifolius Raddi fruits. The purified biflavonoids were characterized in terms of their antibacterial effects. We found that the activity of biflavonoids, including Agathisflavone (AGF), amentoflavone (AMF), and tetrahydroamentoflavone (THAF), was dependent on their chemical configuration and degree of oxidation, with THAF showing the highest activity on planktonic cells. Additionally, biofilm formation and composition were strongly influenced by THAF. Even slight differences in the chemical structure have fundamental effects on the activity of isolated biflavonoids. This suggests a specific binding of these substances in bacteria and thus enables detailed investigations of the mode of action in the future.


