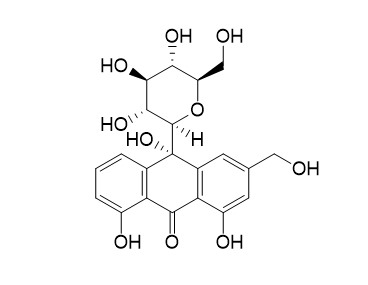
10-Hydroxyaloin BCAS# 134863-92-6 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 134863-92-6 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C21H22O10 | M.Wt | 434.4 |
| Type of Compound | Anthraquinones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

10-Hydroxyaloin B Dilution Calculator

10-Hydroxyaloin B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.302 mL | 11.5101 mL | 23.0203 mL | 46.0405 mL | 57.5506 mL |
| 5 mM | 0.4604 mL | 2.302 mL | 4.6041 mL | 9.2081 mL | 11.5101 mL |
| 10 mM | 0.2302 mL | 1.151 mL | 2.302 mL | 4.6041 mL | 5.7551 mL |
| 50 mM | 0.046 mL | 0.2302 mL | 0.4604 mL | 0.9208 mL | 1.151 mL |
| 100 mM | 0.023 mL | 0.1151 mL | 0.2302 mL | 0.4604 mL | 0.5755 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 10-Methoxygambogenic acid
Catalog No.:BCN0822
CAS No.:2095102-72-8
- 3beta-Hydroxyurs-12,18-dien-28-oic acid beta-D-glucopyranosyl ester
Catalog No.:BCN0821
CAS No.:434942-42-4
- 3beta-(alpha-L-Arabinopyranosyloxy)urs-12,18-dien-28-oic acid beta-D-glucopyranosyl ester
Catalog No.:BCN0820
CAS No.:435269-07-1
- Barbaloin-related compound B
Catalog No.:BCN0819
CAS No.:473225-22-8
- Barbaloin-related compound A
Catalog No.:BCN0818
CAS No.:473225-21-7
- Dehydrodicatechin A
Catalog No.:BCN0817
CAS No.:36048-23-4
- Senkyunolide N
Catalog No.:BCN0816
CAS No.:140694-58-2
- Cajanine
Catalog No.:BCN0815
CAS No.:87402-84-4
- 4-Methoxybenzoylacetic acid
Catalog No.:BCN0814
CAS No.:13422-77-0
- Elgonica dimer A
Catalog No.:BCN0813
CAS No.:132210-48-1
- Rhusflavanone
Catalog No.:BCN0811
CAS No.:53060-72-3
- Agathisflavone
Catalog No.:BCN0810
CAS No.:28441-98-7
- Gambogic acid A
Catalog No.:BCN0824
CAS No.:1592842-93-7
- 10-Carboxyloganin
Catalog No.:BCN0825
CAS No.:182172-02-7
- Oblonganoside D
Catalog No.:BCN0826
CAS No.:943021-91-8
- Cannabisin P
Catalog No.:BCN0827
CAS No.:2756983-19-2
- Pinocembrin 7-O-(4'',6''-hexahydroxydiphenoyl)-beta-D-glucose
Catalog No.:BCN0828
CAS No.:1825287-22-6
- Oxytroflavoside E
Catalog No.:BCN0829
CAS No.:1391144-84-5
- 12beta-Acetoxy-3beta-hydroxy-7,11,15,23-tetraoxo-lanost-8,20-diene-26-oic acid
Catalog No.:BCN0830
CAS No.:1085338-75-5
- Oxytroflavoside A
Catalog No.:BCN0831
CAS No.:1391144-80-1
- Gardoside methyl ester
Catalog No.:BCN0832
CAS No.:93930-20-2
- Ganoderenic acid K
Catalog No.:BCN0833
CAS No.:942950-94-9
- 2,6-Dihydroxyacetophenone-4-O-[4',6'-(S)-hexahydroxydiphenoyl]-beta-D-glucose
Catalog No.:BCN0834
CAS No.:1781226-44-5
- Ganolucidic acid D
Catalog No.:BCN0835
CAS No.:102607-22-7
Identification of major metabolites in Aloe littoralis by high-performance liquid chromatography-nuclear magnetic resonance spectroscopy.[Pubmed:14515998]
Phytochem Anal. 2003 Sep-Oct;14(5):275-80.
Examination of the leaf exudate of the South African species Aloe littoralis by reversed-phase HPLC revealed the presence of two major metabolites. The identification of the two compounds without isolation was attempted by HPLC-NMR based on separation using a C18 column eluting with a deuterium oxide:acetonitrile solvent gradient and an inverse HPLC-NMR probe. For each compound, one-dimensional proton spectra, and two-dimensional homonuclear COSY and TOCSY, and heteronuclear HSQC and HMBC, spectra were collected. On the basis of the data obtained, the metabolites were characterised as 10-Hydroxyaloin B and deacetyllittoraloin.
10-Hydroxyaloin B 6'-O-Acetate, an Oxanthrone from Aloe claviflora[Pubmed:9548856]
J Nat Prod. 1998 Feb 27;61(2):256-7.
Analysis of the leaf exudate of Aloe claviflora resulted in the isolation of a new oxanthrone, 10-Hydroxyaloin B 6'-O-acetate (1), whose structure was determined on the basis of spectral evidence as well as by conversion to the known compound 10-Hydroxyaloin B (2).
Structure revision of 4-hydroxyaloin: 10-hydroxyaloins a and B as main in vitro-oxidation products of the diastereomeric aloins1.[Pubmed:17226466]
Planta Med. 1992 Jun;58(3):259-62.
A reinvestigation of the anthrone derivative 4-hydroxyaloin as main product of the mild IN VITRO-oxidation of aloin has led to the revision of the proposed structure as the aloin-analogous oxanthrone derivative, the new 10-hydroxyaloin, which was prepared from aloin by an improved procedure in ammonia at pH 9. 10-Hydroxyaloin was separated into its C10-diastereomers A and B by analytical and preparative chromatographic methods. Their structures were elucidated by spectroscopic methods (FAB-MS; (1)H/ (13)C-NMR; CD), which show that 10-hydroxyaloin A is the 10 R,1' R compound and that 10-Hydroxyaloin B has the 10 S,1' R-configuration.


