Camellianin BCAS# 109232-76-0 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 109232-76-0 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
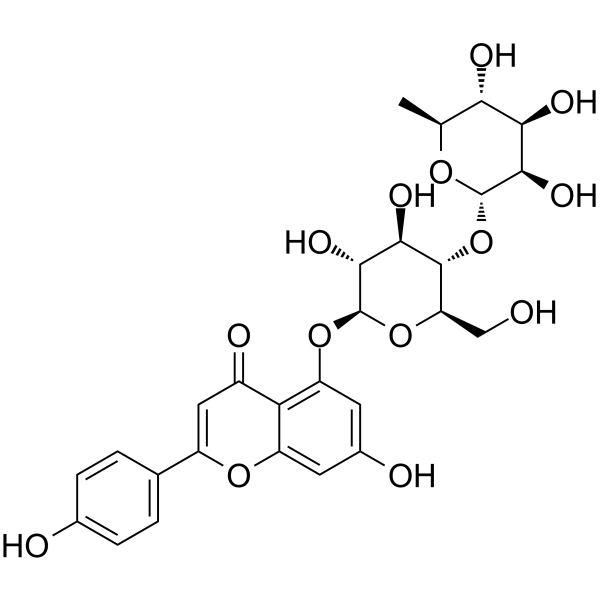
| Formula | C27H30O14 | M.Wt | 578.52 |
| Type of Compound | Flavones/Flavanones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Camellianin B Dilution Calculator

Camellianin B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7285 mL | 8.6427 mL | 17.2855 mL | 34.571 mL | 43.2137 mL |
| 5 mM | 0.3457 mL | 1.7285 mL | 3.4571 mL | 6.9142 mL | 8.6427 mL |
| 10 mM | 0.1729 mL | 0.8643 mL | 1.7285 mL | 3.4571 mL | 4.3214 mL |
| 50 mM | 0.0346 mL | 0.1729 mL | 0.3457 mL | 0.6914 mL | 0.8643 mL |
| 100 mM | 0.0173 mL | 0.0864 mL | 0.1729 mL | 0.3457 mL | 0.4321 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Protoneogracillin
Catalog No.:BCX1643
CAS No.:191334-50-6
- 2,4,6-trichlorol-3-methyl-5-methoxyphenol-1-O-β-D-glucopyranosyl-(1→6)-β-D-glucopyranoside
Catalog No.:BCX1642
CAS No.:1447969-66-5
- (2,4,6-trichloro-3-hydroxy-5-methoxyphenyl)methyl β-D-glucopyranoside
Catalog No.:BCX1641
CAS No.:2839363-14-1
- 4-chloro-3-methoxy-5-methylphenyl 6-O-(6-deoxy-β-L-mannopyranosyl)-β-D-glucopyranoside
Catalog No.:BCX1640
CAS No.:2839363-15-2
- (2,4-dichloro-3,5-dimethoxyphenyl)methyl 6-O-β-D-glucopyranosyl-β-D-glucopyranoside
Catalog No.:BCX1639
CAS No.:2739844-79-0
- 1,3-Dilinoleoyl-2-oleoylglycerol
Catalog No.:BCX1638
CAS No.:2190-22-9
- 10-O-[(E)-3,4-Dimethoxycinnamoyl]-catalpol
Catalog No.:BCX1637
CAS No.:834155-36-1
- 10-O-trans-p-methoxycinnamoyl-catalpol
Catalog No.:BCX1636
CAS No.:201605-27-8
- BioCrick001
Catalog No.:BCX1635
CAS No.:2600799-45-7
- Epiberberine chloride
Catalog No.:BCX1634
CAS No.:889665-86-5
- (7aR,8R,13aR,13bS,13cR)-Dodecahydro-8-hydroxy-1H,5H,10H-dipyrido[2,1-f:3',2',1'-ij][1,6]naphthyridin...
Catalog No.:BCX1633
CAS No.:2306139-04-6
- 2-[3-(3,7-Dimethyl-2,6-octadien-1-yl)-2,4-dihydroxyphenyl]-5,7-dihydroxy-4H-1-benzopyran-4-one
Catalog No.:BCX1632
CAS No.:2226467-12-3
- n-Octadecane
Catalog No.:BCX1645
CAS No.:593-45-3
- Tellimagrandin II
Catalog No.:BCX1646
CAS No.:81571-72-4
- Norswertianin
Catalog No.:BCX1647
CAS No.:22172-15-2
- Wilfordinine D
Catalog No.:BCX1648
CAS No.:256937-98-1
- 1-Methyl-2-[(4Z,7Z)-4,7-tridecadienyl]-4(1H)-quinolone
Catalog No.:BCX1649
CAS No.:120693-53-0
- 1-Methyl-2-[(6Z,9Z)-6,9-pentadecadiene]-4(1H)-quinolone
Catalog No.:BCX1650
CAS No.:120693-52-9
- β-D-glucopyranosyl 3-O-[(O-β-D-xylopyranosyl-(1→2) (O-α-L-rhamnopyranosyl(1→3)β-D-glucuronopyranosyl...
Catalog No.:BCX1651
CAS No.:111567-21-6
- Erythrosin B
Catalog No.:BCX1652
CAS No.:568-63-8
- Entadamide A 2' -O- ( 6''-O-β-D-glucopyranosyl ) -β-D-glucopyranoside
Catalog No.:BCX1653
CAS No.:1427191-48-7
- Tetrahydrojatrorrhizine
Catalog No.:BCX1654
CAS No.:27313-86-6
- (-)-(E)-a-Atlantone
Catalog No.:BCX1655
CAS No.:108645-54-1
- 4-Isopropyl-6-methyl-1-tetralone
Catalog No.:BCX1656
CAS No.:1270089-06-9
Synthetic investigation toward apigenin 5-O-glycoside camellianin B as well as the chemical structure revision.[Pubmed:27145917]
Org Biomol Chem. 2016 Jun 7;14(21):4842-7.
Capitalizing on the Au(i)-catalyzed ortho-alkynylbenzoate glycosylation method, the first total synthesis of the proposed structure of apigenin-5-O-glycoside Camellianin B was achieved, wherein three approaches, one linear and two convergent, were established, through which the synthetic structures were firmly corroborated. Meanwhile, through the synthesis of anthentic Camellianin B via commercially available camellianin A, the misassigned structures of camellianins A and B were revised.
Pharmacokinetics and tissue distribution study of camellianin A and its major metabolite in rats by liquid chromatography with tandem mass spectrometry.[Pubmed:26117310]
J Chromatogr B Analyt Technol Biomed Life Sci. 2015 Aug 1;997:200-9.
Camellianin A is a major active constituent of Adinandra nitida. A LC-MS/MS method for the determination of camellianin A and its metabolite (Camellianin B) in rat plasma and tissues was developed and applied to a pharmacokinetics and tissue distribution study. Samples were separated on a Waters HSS T3 column with a mobile phase consisted of methanol and water (containing 0.1% formic acid). MS/MS detection was carried out on a triple-quadruple mass spectrometer under negative ESI mode. Pharmacokinetics study showed that camellianin A was rapidly eliminated with a t1/2 of 92.6+/-41.4h and CL of 3.19+/-0.471L/min/kg. Additionally, camellianin A showed a low oral bioavailability of 2.99% and a narrow tissue distribution; however, Camellianin B was proved to have a wide tissue distribution with brain penetration. The data presented in this study provides useful information for the further applications of A. nitida and camellianin A.
[Simultaneous determination of five flavonoid compounds in leaves of Adinandra nitida by HPLC-PAD].[Pubmed:21141488]
Zhongguo Zhong Yao Za Zhi. 2010 Sep;35(18):2406-9.
OBJECTIVE: To established a novel HPLC-PAD method for the simultaneous determination of five compounds (camellianin A, Camellianin B, apigenin, quercitrin, epicatechin) in leaves of Adinandra nitida. METHOD: These compounds were effectively separated on a reversed-phase C18 column (4.6 mm x 250 mm, 10 microm) with the column temperature at 25 degrees C. The mobile phase was composed of 0.1% aqueous formic acid and methanol. The flow rate was 1.0 mL x min(-1), the detection wavelength was set at 280 nm before 15 min for detecting epicatechin and at 262 nm from 15 to 40 min for detecting the rest four compounds. RESULT: All the linearities were good (r > 0.9991) within their test ranges. The established method showed good precision with overall intra-day and interday RSD values less than 3.33% and 3.2%, respectively. The average recoveries were in the range of 96.08% to 100.30% with RSD from 2.0% to 4.0%. The limits of detection (LOD) and quantification (LOQ) in the range of ranged from 0.9 to 5.6 ng and 3.0 to 19.0 ng, respectively. CONCLUSION: This established method can be applied to evaluate the intrinsic quality of the leaves of A. nitida.
Antioxidant and angiotensin converting enzyme (ACE) inhibitory activities of ethanol extract and pure flavonoids from Adinandra nitida leaves.[Pubmed:20738217]
Pharm Biol. 2010 Dec;48(12):1432-8.
CONTEXT: Adinandra nitida Merr. ex. H.L. Li (Theaceae) is an indigenous plant in south China. Its leaves have been reported to have many curative effects such as reducing blood pressure, as well as antibacterial, antioxidant, and analgesic properties, which could be used in foods and medicines. OBJECTIVE: The antioxidant and angiotensin converting enzyme (ACE) inhibitory activities of the main flavonoids and ethanol extract (EE) of A. nitida leaves were investigated for the first time. MATERIALS AND METHODS: The main flavonoids of A. nitida leaves (camellianin A, Camellianin B) were prepared and their contents in EE were determined by HPLC. The antioxidant activities of the samples were measured by DPPH radical scavenging assay and Rancimat test. The ACE inhibitory activities of the samples were carried out by using an assay kit with hippuryl-glycyl-glycine as substrate. RESULTS: The contents of camellianin A, Camellianin B and apigenin in EE were determined as 41.98, 2.67, and 1.73%, respectively. The antioxidant activities of the flavonoids were far lower than that of EE in DPPH radical scavenging and Rancimat assays. However, the ACE-inhibitory activities of camellianin A, Camellianin B and apigenin were higher than that of EE. DISCUSSION AND CONCLUSION: The flavonoid content of EE was more than 45%. The high activities of EE in DPPH scavenging and Rancimat assay could be mainly attributed to compounds other than flavonoids. However, the ACE-inhibitory activity of EE could be mainly attributed to the presence of the flavonoids.


