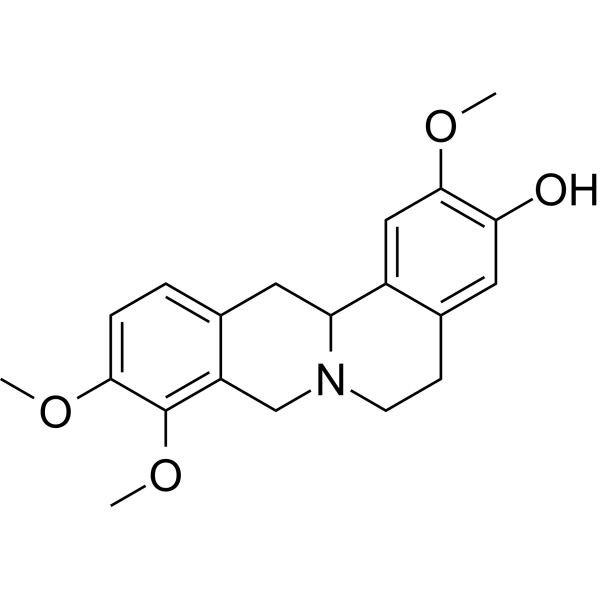
TetrahydrojatrorrhizineCAS# 27313-86-6 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 27313-86-6 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C20H23NO4 | M.Wt | 341.4 |
| Type of Compound | Quinolines/Isoquinolines | Storage | Desiccate at -20°C |
| Synonyms | (R,S)-Tetrahydrojatrorrhizine,(±)-Corypalmine,(±)-Discretinine,(±)-Tetrahydrojatrorrhizine,2,9,10-Tr... | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Tetrahydrojatrorrhizine Dilution Calculator

Tetrahydrojatrorrhizine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9291 mL | 14.6456 mL | 29.2912 mL | 58.5823 mL | 73.2279 mL |
| 5 mM | 0.5858 mL | 2.9291 mL | 5.8582 mL | 11.7165 mL | 14.6456 mL |
| 10 mM | 0.2929 mL | 1.4646 mL | 2.9291 mL | 5.8582 mL | 7.3228 mL |
| 50 mM | 0.0586 mL | 0.2929 mL | 0.5858 mL | 1.1716 mL | 1.4646 mL |
| 100 mM | 0.0293 mL | 0.1465 mL | 0.2929 mL | 0.5858 mL | 0.7323 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Entadamide A 2' -O- ( 6''-O-β-D-glucopyranosyl ) -β-D-glucopyranoside
Catalog No.:BCX1653
CAS No.:1427191-48-7
- Erythrosin B
Catalog No.:BCX1652
CAS No.:568-63-8
- β-D-glucopyranosyl 3-O-[(O-β-D-xylopyranosyl-(1→2) (O-α-L-rhamnopyranosyl(1→3)β-D-glucuronopyranosyl...
Catalog No.:BCX1651
CAS No.:111567-21-6
- 1-Methyl-2-[(6Z,9Z)-6,9-pentadecadiene]-4(1H)-quinolone
Catalog No.:BCX1650
CAS No.:120693-52-9
- 1-Methyl-2-[(4Z,7Z)-4,7-tridecadienyl]-4(1H)-quinolone
Catalog No.:BCX1649
CAS No.:120693-53-0
- Wilfordinine D
Catalog No.:BCX1648
CAS No.:256937-98-1
- Norswertianin
Catalog No.:BCX1647
CAS No.:22172-15-2
- Tellimagrandin II
Catalog No.:BCX1646
CAS No.:81571-72-4
- n-Octadecane
Catalog No.:BCX1645
CAS No.:593-45-3
- Camellianin B
Catalog No.:BCX1644
CAS No.:109232-76-0
- Protoneogracillin
Catalog No.:BCX1643
CAS No.:191334-50-6
- 2,4,6-trichlorol-3-methyl-5-methoxyphenol-1-O-β-D-glucopyranosyl-(1→6)-β-D-glucopyranoside
Catalog No.:BCX1642
CAS No.:1447969-66-5
- (-)-(E)-a-Atlantone
Catalog No.:BCX1655
CAS No.:108645-54-1
- 4-Isopropyl-6-methyl-1-tetralone
Catalog No.:BCX1656
CAS No.:1270089-06-9
- 2,2-Diphenyl-1-picrylhydrazyl radical(DPPH)
Catalog No.:BCX1657
CAS No.:1898-66-4
- 1,2,3,7-Tetrahydroxy-8-methoxy-6-methyl-9,10-anthracenedione
Catalog No.:BCX1658
CAS No.:130018-57-4
- (E)-Isoconiferin (Citrusin D)
Catalog No.:BCX1659
CAS No.:113349-27-2
- lagunamine
Catalog No.:BCX1660
CAS No.:126721-42-4
- Podecdysone C
Catalog No.:BCX1661
CAS No.:19458-46-9
- Glucodichotomine B
Catalog No.:BCX1662
CAS No.:845673-16-7
- Rubrofusarin-6-O-β-D-glucopyranoside
Catalog No.:BCX1663
CAS No.:132922-80-6
- (1E)-3-Methoxy-8,12-epoxygermacra-1,7,10,11-tetraen-6-one
Catalog No.:BCX1664
CAS No.:383368-26-1
- (-)-10-epi-α-Cyperone
Catalog No.:BCX1665
CAS No.:2303-31-3
- Inonotusol F
Catalog No.:BCX1666
CAS No.:1534433-74-3
Evaluation of raw and processed Phellodendri Chinensis Cortex using the quality marker analysis strategy by UHPLC-Q-Orbitrap MS and multivariate statistical analysis.[Pubmed:37583566]
Front Chem. 2023 Jul 31;11:1223865.
Introduction: Phellodendri Chinensis Cortex is a necessary part of healthcare for its significant clinical efficacy. Raw and processed Phellodendri Chinensis Cortex is both documented in the Chinese Pharmacopoeia (2015). After processing, the therapeutic effects are believed to differ according to traditional Chinese medicine theories. However, the chemical mechanism responsible for this processing, according to traditional Chinese medicine theories, is still not clear. Methods: In this study, the therapeutic effects of various ions were examined based on traditional Chinese medicine theories by ultra-high performance liquid chromatography-hybrid quadrupole-Orbitrap mass spectrometry (UHPLC-Q-Orbitrap MS) coupled with multivariate statistical analysis, such as principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA), to comprehensively compare the differences between raw and processed Phellodendri Chinensis Cortex for the first time. Results: A total of 48 compounds were screened, out and 10 of them simultaneously transformed with significant variation in processed products compared with raw materials. It was illustrated that the contents of berberine, palmatine, jatrorrhizine, magnoflorine, menisperine, phellodendrine, Tetrahydrojatrorrhizine, and tetrahydropalmatine decreased, while the compounds of berberrubine and fernloylquinic acid methyl ester newly appeared in processed herbs. This is likely to be related to the conversion of ingredients during processing. Discussion: Altogether, the fact that quality markers have been successfully identified to differentiate processed Phellodendri Chinensis Cortex from raw materials suggests that this approach could be used for the investigation of chemical transformation mechanisms involved in the processing of herbal medicine.
An ultra-high-performance liquid chromatography-tandem mass spectrometry method for the simultaneous quantitation of 10 alkaloids of Corydalis Decumbentis Rhizoma preparation in dog plasma and its application to a pharmacokinetic study.[Pubmed:36303263]
Basic Clin Pharmacol Toxicol. 2023 Jan;132(1):33-50.
OBJECTIVE: A sensitive and rapid ultra-high-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) method was successfully applied to the determination of 10 alkaloids in beagle dog plasma following a single oral dose of Xiatianwu capsules and enteric-coated capsules, with theophylline serving as the internal standard (IS). METHODS: Plasma samples were preprocessed using liquid-liquid extraction (LLE) with ethyl acetate ahead to separation using a gradient elution procedure on a Waters ACQUITY UPLC HSS T3 column (1.8 mum, 100 x 2.1 mm). The mobile phase was composed of 0.1% formic acid solution and acetonitrile at the flow rate of 0.3 ml/min. Multiple reaction monitoring (MRM) was used to determine the analytes in the positive ion mode. RESULTS: The calibration curves for 10 analytes demonstrated a high degree of linearity (r >/= 0.9920). The lower limit of quantification (LLOQ) values for 10 alkaloids were all more than 1.074 ng/ml, and matrix effects varied from 94.25% to 106.15%. The mean extraction recovery of quality control samples at low (LQC), medium (MQC) and high (HQC) concentrations, as well as IS, was all more than 76.60%. The intra-day and inter-day precision (RSD) also satisfied the requirement. Simultaneously, the variation of assay accuracies (RE) was between 13.05% and 9.38%. CONCLUSION: The test was validated in accordance with regulatory bioanalytical guidelines and was found to be suitable for use in a pharmacokinetic investigation of these compounds in beagle dogs after oral administration of Xiatianwu general capsules and enteric-coated capsules. The C(max) of 10 alkaloids ranged from 52.61 to 192.46 ng/ml after oral administration of Xiatianwu capsules, and from 67.50 to 247.36 ng/ml. The T(max) was between 0.59 and 1.33 h of Xiatianwu capsules, and between 1.08 and 2.00 h of enteric-coated capsules. The t(1/2) ranged from 3.18 to 7.47 h of general capsules, and from 6.01 to 11.36 h. AUC(0-t) ranged from 181.06 to 722.74 ng.h/ml of Xiatianwu capsules, and from 275.03 to 884.17 ng.h/ml of enteric-coated capsules. The C(max) of enteric-coated capsules were significantly increased except for tetrahydropalmatine and berberine. T(max) of general capsules were less than 1 h, and of enteric-coated capsules were less than 2 h. The t(1/2) of dehydrocorydaline, palmatine, Tetrahydrojatrorrhizine, jatrorrhizine and coptisine in enteric-coated capsules was longer than that in ordinary capsule. The AUC(0-t) and AUC(0-infinity) of bicuculline, dehydrocorydaline, protopine, magnoflorine, Tetrahydrojatrorrhizine, jatrorrhizine, berberine and coptisine were all significantly higher in enteric-coated capsules.
Tetrahydroprotoberberine and aporphine alkaloids from rollinia leptopetala.[Pubmed:21214484]
Pharm Biol. 2000;38(4):318-20.
Tetrahydrojatrorrhizine, discretamine, anonaine and roemerine were isolated from the roots of Rollinia leptopetala R. E. Fries. They were identified and characterized by nuclear magnetic resonance spectroscopy ( 1 H and 13 C) with the aid of two-dimensional techniques ( 1 H- 1 H COSY, HETCOR, HMQC and HMBC). With the exception of Tetrahydrojatrorrhizine, the presence of these alkaloids in R. leptopetala has not been reported previously.
Isolation and Characterization of S-Adenosyl-L-Methionine:Tetrahydroberberine-cis-N-Methyltransferase from Suspension Cultures of Sanguinaria canadensis L.[Pubmed:12232209]
Plant Physiol. 1994 May;105(1):395-403.
As part of a continuing study of the induction of alkaloid biosynthesis, we report the isolation to homogeneity and characterization of S-adenosyl-L-methionine:tetrahydroberberine-cis-N-mehtyltransferase from suspension cultures of Sanguinaria canadensis that were induced to produce alkaloids by hormone depletion. This enzyme catalyzes the stereospecific transfer of a methyl group from S-adenosyl-L-methionine to the tertiary nitrogen of the protoberberine alkaloid tetrahydroberberine (canadine). The enzyme was purified 315-fold by ammonium sulfate precipitation, gel permeation chromatography, affinity dye chromatography, and both diethylaminoethyl and Mono-Q ion-exchange chromatography. The enzyme was further purified to an optimum specific activity of 225 nkat/mg of protein (3500-fold) and electrophoretic homogeneity by native polyacrylamide gel electrophoresis (PAGE). In contrast to previous reports with partially purified enzyme, the isolated protein was found to have a pH optimum of 7.0, a temperature optimum of 25 to 30[deg]C, and an isoelectric point of 5.1. Furthermore, the molecular weight of the homogeneous protein was found to be 39,000 by sodium dodecyl sulfate-PAGE. The homogeneous enzyme preferred tetrahydroberberine over all other substrates tested, showing an apparent Km of 2.1 [mu]M, but also showed partial activity with Tetrahydrojatrorrhizine and tetrahydropalmatrubine.


