CatenarinCAS# 476-46-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 476-46-0 | SDF | Download SDF |
| PubChem ID | 10150 | Appearance | Red powder |
| Formula | C15H10O6 | M.Wt | 286.24 |
| Type of Compound | Anthraquinones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
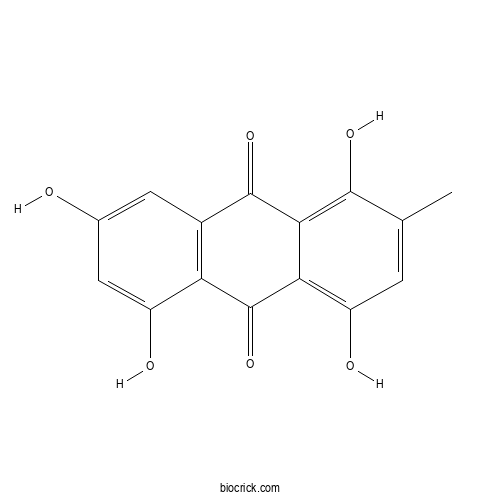
| Chemical Name | 1,4,5,7-tetrahydroxy-2-methylanthracene-9,10-dione | ||
| SMILES | CC1=CC(=C2C(=C1O)C(=O)C3=C(C2=O)C(=CC(=C3)O)O)O | ||
| Standard InChIKey | VWDXGKUTGQJJHJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H10O6/c1-5-2-8(17)11-12(13(5)19)14(20)7-3-6(16)4-9(18)10(7)15(11)21/h2-4,16-19H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Catenarin Dilution Calculator

Catenarin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4936 mL | 17.4679 mL | 34.9357 mL | 69.8714 mL | 87.3393 mL |
| 5 mM | 0.6987 mL | 3.4936 mL | 6.9871 mL | 13.9743 mL | 17.4679 mL |
| 10 mM | 0.3494 mL | 1.7468 mL | 3.4936 mL | 6.9871 mL | 8.7339 mL |
| 50 mM | 0.0699 mL | 0.3494 mL | 0.6987 mL | 1.3974 mL | 1.7468 mL |
| 100 mM | 0.0349 mL | 0.1747 mL | 0.3494 mL | 0.6987 mL | 0.8734 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4-Hydroxythonningianin A
Catalog No.:BCX0540
CAS No.:1055032-77-3
- 22-Hydroxyhopan-2-one
Catalog No.:BCX0539
CAS No.:97230-81-4
- Physcion-10,10'-bianthrone
Catalog No.:BCX0538
CAS No.:21871-90-9
- Variecolorin G
Catalog No.:BCX0537
CAS No.:957780-76-6
- Maltol 3-O-(5-O-p-coumaroyl)-β-D-apiofuranosyl-(1→6)-O-β-D-glucopyranoside
Catalog No.:BCX0536
CAS No.:1018452-31-7
- Eurocristatine
Catalog No.:BCX0535
CAS No.:1459722-08-7
- 1-O-Caffeoyl-3,4-di-O-galloyl-β-D-glucopyranose
Catalog No.:BCX0534
CAS No.:359819-46-8
- 13-Hydroxynootkatone
Catalog No.:BCX0533
CAS No.:141695-86-5
- 1β,8α-Dihydroxyeremophila-7(11),9-dien-12,8-olide
Catalog No.:BCX0532
CAS No.:849700-44-3
- Salcolin A
Catalog No.:BCX0531
CAS No.:1977557-69-9
- 2-Hydroxymethyl-5-isopropylphenol
Catalog No.:BCX0530
CAS No.:111044-81-6
- 4-Hydroxythonningianin B
Catalog No.:BCX0529
CAS No.:2329726-95-4
- Clemoarmanoside B
Catalog No.:BCX0542
CAS No.:915314-08-8
- Sanggenol H
Catalog No.:BCX0543
CAS No.:202526-53-2
- 9''-O-Acetylsalcolin A
Catalog No.:BCX0544
CAS No.:910864-92-5
- 1β-Hydroxy-12-noreremophila-6,9-diene-8,11-dione
Catalog No.:BCX0545
CAS No.:161127-52-2
- (-)-Lyoniresinol 9-O-glucoside
Catalog No.:BCX0546
CAS No.:162613-63-0
- Isophthalic acid
Catalog No.:BCX0547
CAS No.:121-91-5
- 9β-Hydroxynootkatone
Catalog No.:BCX0548
CAS No.:226547-01-9
- Daphnodorin G
Catalog No.:BCX0549
CAS No.:178664-65-8
- meso-Octahydrocurcumin
Catalog No.:BCX0550
CAS No.:135413-63-7
- 1,5-Epoxy-3-hydroxy-1-(4-hydroxy-3,5-dimethoxyphenyl)-7-(4-hydroxy-3-methoxyphenyl)heptane
Catalog No.:BCX0551
CAS No.:719270-40-3
- Daphnodorin H
Catalog No.:BCX0552
CAS No.:178897-27-3
- Berchemol
Catalog No.:BCX0553
CAS No.:126882-59-5
An Update of Anthraquinone Derivatives Emodin, Diacerein, and Catenarin in Diabetes.[Pubmed:34589130]
Evid Based Complement Alternat Med. 2021 Sep 20;2021:3313419.
Diabetes is part of metabolic diseases and is characterized by high blood sugar levels over a prolonged period as result of an insulin-deficient production or an inappropriate response to insulin by our cells. This chronic disease was the direct cause of 1.6 million deaths in 2016 as reported by the World Health Organization. Emodin is a natural product and active ingredient of various Chinese herbs with the chemical formula 1,3,8-trihydroxy-6-methylanthraquinone. Diacerein is another naturally occurring anthraquinone (1,8-diacetoxy-3-carboxyanthraquinone) commonly used as commercial drug to treat osteoarthritis. These two anthraquinone derivatives have been shown to exert antidiabetic activities. Emodin seems to enhance the glucose tolerance and insulin sensibility via activation of PPARgamma and modulation of metabolic-related genes. Diacerein seems to decrease inflammatory cytokines and increase insulin secretion enhancing insulin sensibility and therefore improving glucose control. Other naturally occurring anthraquinone derivatives, such as Catenarin (1,4,6,8-tetrahydroxy-3-methylanthraquinone), have been shown to have antidiabetic activities although few studies have been performed. The synthesis of new emodin derivatives is increasing, but these new molecules have not been tested for diabetes treatment. In the current work, available literature on anthraquinone derivatives' effects in diabetes disease is reviewed. Moreover, we discuss the chemistry, food sources, bioavailability, and toxicity of the naturally occurring anthraquinone with antidiabetic effects.
Secondary Metabolites from the Culture of the Marine Sponge-Associated Fungi Talaromyces tratensis and Sporidesmium circinophorum.[Pubmed:27054912]
Planta Med. 2016 Jun;82(9-10):888-96.
Wortmin (1), meso-1,4-bis(4-methoxybenzyl)-2,3-butanediol (2), and a new isocoumarin derivative tratenopyrone (3) were isolated from the marine sponge-associated fungus Talaromyces tratensis KUFA 0091. A new diphenyl ether derivative, circinophoric acid (4), was isolated, together with the previously reported anthraquinones Catenarin and physcion, the benzophenone monomethylsoluchrin, and beta-ergosterol-5,8-endoperoxide, from the marine sponge-associated fungus Sporidesmium circinophorum KUFA 0043. The structures of the new compounds were established based on an extensive analysis of 1D and 2D NMR spectra, and, in the case of compounds 2-4, also by X-ray analysis. All of the isolated compounds were tested for their antibacterial activity against Gram-positive and Gram-negative bacteria, and multidrug-resistant isolates from the environment, as well as for their anti-quorum sensing based on the pigment production of Chromobacterium violaceum ATCC 31523. None of the compounds exhibited either antibacterial (MIC > 256 microg/mL) or anti-quorum sensing activities. The compounds were also inactive in the antifungal (MIC > 512 microg/mL) and cancer cell line (GI50 > 150 microM) assays.
Naturally occurring anthraquinones: chemistry and therapeutic potential in autoimmune diabetes.[Pubmed:25866536]
Evid Based Complement Alternat Med. 2015;2015:357357.
Anthraquinones are a class of aromatic compounds with a 9,10-dioxoanthracene core. So far, 79 naturally occurring anthraquinones have been identified which include emodin, physcion, cascarin, Catenarin, and rhein. A large body of literature has demonstrated that the naturally occurring anthraquinones possess a broad spectrum of bioactivities, such as cathartic, anticancer, anti-inflammatory, antimicrobial, diuretic, vasorelaxing, and phytoestrogen activities, suggesting their possible clinical application in many diseases. Despite the advances that have been made in understanding the chemistry and biology of the anthraquinones in recent years, research into their mechanisms of action and therapeutic potential in autoimmune disorders is still at an early stage. In this paper, we briefly introduce the etiology of autoimmune diabetes, an autoimmune disorder that affects as many as 10 million worldwide, and the role of chemotaxis in autoimmune diabetes. We then outline the chemical structure and biological properties of the naturally occurring anthraquinones and their derivatives with an emphasis on recent findings about their immune regulation. We discuss the structure and activity relationship, mode of action, and therapeutic potential of the anthraquinones in autoimmune diabetes, including a new strategy for the use of the anthraquinones in autoimmune diabetes.
Catenarin Prevents Type 1 Diabetes in Nonobese Diabetic Mice via Inhibition of Leukocyte Migration Involving the MEK6/p38 and MEK7/JNK Pathways.[Pubmed:22454693]
Evid Based Complement Alternat Med. 2012;2012:982396.
Inflammation contributes to leukocyte migration, termed insulitis, and beta-cell loss in type 1 diabetes (T1D). Naturally occurring anthraquinones are claimed as anti-inflammatory compounds; however, their actions are not clear. This study aimed to investigate the effect and mechanism of Catenarin on the inflammatory disease, T1D. Catenarin and/or its anthraquinone analogs dose-dependently suppressed C-X-C chemokine receptor type 4 (CXCR4)- and C-C chemokine receptor type 5 (CCR5)-implicated chemotaxis in leukocytes. Catenarin, the most potent anthraquinone tested in the study, prevented T1D in nonobese diabetic mice. Mechanistic study showed that Catenarin did not act on the expression of CCR5 and CXCR4. On the contrary, Catenarin inhibited CCR5- and CXCR4-mediated chemotaxis via the reduction of the phosphorylation of mitogen-activated protein kinases (p38 and JNK) and their upstream kinases (MKK6 and MKK7), and calcium mobilization. Overall, the data demonstrate the preventive effect and molecular mechanism of action of Catenarin on T1D, suggesting its novel use as a prophylactic agent in T1D.
Influence of carbon source on growth and mycotoxin production by isolates of Pyrenophora tritici-repentis from wheat.[Pubmed:20962911]
Can J Microbiol. 2010 Oct;56(10):874-84.
The fungus Pyrenophora tritici-repentis can infect wheat kernels, causing red smudge, and has been shown to produce the anthraquinone mycotoxins emodin, Catenarin, and islandicin. The growth of 8 fungal isolates from diverse regions was evaluated on various culture media and was found to be generally slowest on the semisynthetic Fries medium. The choice of carbon source had a significant effect on mycotoxin production, as assessed by high-performance liquid chromatography. The highest emodin concentration (194.18 +/- 16.26 microg/g medium) was observed for isolate Alg 3-24 on Fries medium supplemented with fructose, while the highest Catenarin concentration (302.54 +/-13.92 microg/g medium) was observed for TS93-71B on Fries medium supplemented with starch. Islandicin was not produced by any isolate under the conditions tested. Evaluation of the dynamics of mycotoxin production by isolate 331-2 on V8-potato dextrose agar medium revealed a rapid accumulation of emodin and Catenarin during the first week of incubation, followed by a large decline by 14 days. Differences in the growth of and mycotoxin production by isolates of P. tritici-repentis likely resulted from the differential composition of the media and (or) intraspecies variability. Accordingly, the optimization of growth medium should be considered when evaluating the potential of specific isolates for mycotoxin production.
Influence of water activity and temperature on growth and mycotoxin production by isolates of Pyrenophora tritici-repentis from wheat.[Pubmed:19268381]
Int J Food Microbiol. 2009 May 31;131(2-3):251-5.
Pyrenophora tritici-repentis is a phytopathogenic fungus that can infect wheat kernels and leaves, causing red smudge and tan spot, respectively. A number of P. tritici-repentis isolates have been shown to be mycotoxigenic, producing the anthraquinone mycotoxins emodin, Catenarin and islandicin. The influence of water activity (a(w); 0.75-0.99 a(w)) and temperature (5-45 degrees C) on growth and mycotoxin production by five isolates of P. tritici-repentis was studied. All isolates grew at 0.95-0.99 a(w) and 15-25 degrees C on a wheat-based medium, with three isolates also producing small colonies at 5 degrees C. The optimal growth conditions for all isolates consisted of 0.99 a(w) and 25 degrees C, and growth was significantly reduced at 0.95 a(w) and/or 15 degrees C. Emodin and Catenarin were detected in cultures of all isolates, at concentrations ranging from 0.06+/-0.04 to 11.31+/-2.96 microg emodin/g medium, and from 0.09+/-0.06 to 53.42+/-4.36 microg Catenarin/g medium. In most isolates, the concentrations of emodin and Catenarin declined under suboptimal growth conditions. However, in some isolates, significant increases in the concentrations of both compounds were observed under suboptimal conditions. Islandicin was detected in cultures of only three isolates, at concentrations ranging from 0.07+/-0.05 to 5.69+/-0.76 microg/g medium. The results suggest that growth and mycotoxin formation by P. tritici-repentis are markedly influenced by a(w) and temperature, and that this fungus is hygrophilic. Therefore, infection and contamination of kernels by P. tritici-repentis are likely to occur in the field rather than in storage. To our knowledge, this is the first study on the effect of environmental factors on mycelial growth and mycotoxin production by P. tritici-repentis.
[Study on the chemical constituents of Rhizoma Cyperi].[Pubmed:18973011]
Zhong Yao Cai. 2008 Jul;31(7):990-2.
OBJECTIVE: To study the chemical constituents of Rhizoma Cyperi. METHODS: The constituents were separated and purified by silica gel column chromatography, their structures were identified on the basis of physico-chemical properties and spectral data. RESULTS: Six compounds were isolated and identified as physicion (1), hexadecanoic acid (2), beta-sitosterol (3), stigmasterol (4), Catenarin (5), daucosterol (6). CONCLUSION: Compounds 1, 4, 5 were isolated from this plant for the first fime.
Cytotoxic principles from Ventilago leiocarpa.[Pubmed:11374975]
J Nat Prod. 2001 May;64(5):674-6.
Three new anthraquinones, islandicin 4-methyl ether (1), 1,2,6-trihydroxy-7,8-dimethoxy-3-methylanthraquinone (2), and 2-hydroxyemodin 1-methyl ether (3) as well as two known triterpenoids [taraxerol (4), lupeol (5)], six anthraquinones [chrysophanol (6), islandicin (8), parietin (9), emodin (10), Catenarin (11), skyrin (15)], a 2,3-dihydroflavonol [(+)-aromadendrin (12)], two benzisochromanquinones [ventiloquinone K (13) and ventiloquinone I (14)], and stigmasterol (7) were isolated from Ventilago leiocarpa. The cytotoxicity of these compounds to various tumor cell lines was evaluated, and compound 15 significantly suppressed growth of HeLa, Vero, K562, Raji, Wish, and Calu-1 tumor cell lines. With the exception of K562 cells, the proliferation of other tumor cell lines was inhibited by compounds 3 and 10.
Metabolic products of microorganisms. 185. The anthraquinones of the Aspergillus glaucus group. I. Occurrence, isolation, identification and antimicrobial activity.[Pubmed:7406630]
Arch Microbiol. 1980 Jul;126(3):223-30.
The occurrence of emodin, erythroglaucin, physcion, physcion-9-anthrone, questin, Catenarin, and Catenarin-8-methyl ether in different species of the Aspergillus glaucus group (genus Eurotium) was investigated. So far Catenarin-8-methyl ether (1, 4, 6-trihydroxy-8-methoxy-3-methylanthraquinone) has not been described as a natural product; it was therefore given the name rubrocristin. The chemical and physical properties of rubrocristin are reported. In addition a new violet pigment (C16H12O5) was isolated and characterized by its MS-, IR- and UV-spectra. The antimicrobial properties of all substances were examined in the agar diffusion assay. Gram-positive bacteria were the most sensitive organisms and Catenarin was the most active naturally occurring substance. Synthetically obtained 1, 4, 6, 8-tetrahydroxyanthraquinone was slightly more active than Catenarin, whereas rubrocristin showed no antibacterial activity.


