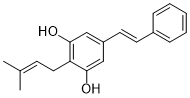
Chiricanine ACAS# 350593-30-5 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 350593-30-5 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C19H20O2 | M.Wt | 280.4 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Chiricanine A Dilution Calculator

Chiricanine A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5663 mL | 17.8317 mL | 35.6633 mL | 71.3267 mL | 89.1583 mL |
| 5 mM | 0.7133 mL | 3.5663 mL | 7.1327 mL | 14.2653 mL | 17.8317 mL |
| 10 mM | 0.3566 mL | 1.7832 mL | 3.5663 mL | 7.1327 mL | 8.9158 mL |
| 50 mM | 0.0713 mL | 0.3566 mL | 0.7133 mL | 1.4265 mL | 1.7832 mL |
| 100 mM | 0.0357 mL | 0.1783 mL | 0.3566 mL | 0.7133 mL | 0.8916 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- cis-ε-Viniferin
Catalog No.:BCN0640
CAS No.:62250-12-8
- Gingerenone A
Catalog No.:BCN0639
CAS No.:128700-97-0
- (3S,5S)-[6]-Gingerdiol
Catalog No.:BCN0638
CAS No.:143615-76-3
- 4-(4-Hydroxy-3-methoxyphenyl)butane-1,2-diol
Catalog No.:BCN0637
CAS No.:39115-22-5
- Bisisorhapontigenin B
Catalog No.:BCN0636
CAS No.:
- Isocalophyllic acid
Catalog No.:BCN0635
CAS No.:157810-76-9
- Calophyllic acid
Catalog No.:BCN0634
CAS No.:36626-19-4
- (3S,5S)-[4]-Gingerdiol
Catalog No.:BCN0633
CAS No.:1448789-37-4
- Annphenone
Catalog No.:BCN0632
CAS No.:61775-18-6
- Colelomycerone A
Catalog No.:BCN0631
CAS No.:1191896-73-7
- Campsiketalin
Catalog No.:BCN0630
CAS No.:93675-96-8
- Ethyl 13-hydroxy-α-linolenate
Catalog No.:BCN0629
CAS No.:123435-84-7
- Gneafricanin F
Catalog No.:BCN0642
CAS No.:477561-12-9
- Nandinaside A
Catalog No.:BCN0643
CAS No.:1813517-23-5
- [6]-Gingerdiol
Catalog No.:BCN0644
CAS No.:154905-69-8
- [4]-Gingerol
Catalog No.:BCN0645
CAS No.:41743-68-4
- Nujiangefolin A
Catalog No.:BCN0646
CAS No.:1393644-51-3
- 1,7-Bis(3,4-dihydroxyphenyl)heptane-3,5-diyl diacetate
Catalog No.:BCN0647
CAS No.:138870-97-0
- 3''-Demethylhexahydrocurcumin
Catalog No.:BCN0648
CAS No.:881008-71-5
- Cannabisin C
Catalog No.:BCN0649
CAS No.:
- Benzyl β-D-glucopyranoside
Catalog No.:BCN0650
CAS No.:4304-12-5
- Homovanillyl alcohol 4-O-glucoside
Catalog No.:BCN0651
CAS No.:104380-15-6
- Dihydroxyalnusone
Catalog No.:BCN0652
CAS No.:160507-18-6
- Eulophiol
Catalog No.:BCN0653
CAS No.:87402-72-0
Suppression of Aflatoxin Production in Aspergillus Species by Selected Peanut (Arachis hypogaea) Stilbenoids.[Pubmed:29207242]
J Agric Food Chem. 2018 Jan 10;66(1):118-126.
Aspergillus flavus is a soil fungus that commonly invades peanut seeds and often produces carcinogenic aflatoxins. Under favorable conditions, the fungus-challenged peanut plant produces and accumulates resveratrol and its prenylated derivatives in response to such an invasion. These prenylated stilbenoids are considered peanut antifungal phytoalexins. However, the mechanism of peanut-fungus interaction has not been sufficiently studied. We used pure peanut stilbenoids arachidin-1, arachidin-3, and Chiricanine A to study their effects on the viability of and metabolite production by several important toxigenic Aspergillus species. Significant reduction or virtually complete suppression of aflatoxin production was revealed in feeding experiments in A. flavus, Aspergillus parasiticus, and Aspergillus nomius. Changes in morphology, spore germination, and growth rate were observed in A. flavus exposed to the selected peanut stilbenoids. Elucidation of the mechanism of aflatoxin suppression by peanut stilbenoids could provide strategies for preventing plant invasion by the fungi that produce aflatoxins.
A Stilbenoid-Specific Prenyltransferase Utilizes Dimethylallyl Pyrophosphate from the Plastidic Terpenoid Pathway.[Pubmed:27356974]
Plant Physiol. 2016 Aug;171(4):2483-98.
Prenylated stilbenoids synthesized in some legumes exhibit plant pathogen defense properties and pharmacological activities with potential benefits to human health. Despite their importance, the biosynthetic pathways of these compounds remain to be elucidated. Peanut (Arachis hypogaea) hairy root cultures produce a diverse array of prenylated stilbenoids upon treatment with elicitors. Using metabolic inhibitors of the plastidic and cytosolic isoprenoid biosynthetic pathways, we demonstrated that the prenyl moiety on the prenylated stilbenoids derives from a plastidic pathway. We further characterized, to our knowledge for the first time, a membrane-bound stilbenoid-specific prenyltransferase activity from the microsomal fraction of peanut hairy roots. This microsomal fraction-derived resveratrol 4-dimethylallyl transferase utilizes 3,3-dimethylallyl pyrophosphate as a prenyl donor and prenylates resveratrol to form arachidin-2. It also prenylates pinosylvin to Chiricanine A and piceatannol to arachidin-5, a prenylated stilbenoid identified, to our knowledge, for the first time in this study. This prenyltransferase exhibits strict substrate specificity for stilbenoids and does not prenylate flavanone, flavone, or isoflavone backbones, even though it shares several common features with flavonoid-specific prenyltransferases.
Total synthesis of chiricanine A, arahypin-1, trans-arachidin-2, trans-arachidin-3, and arahypin-5 from peanut seeds.[Pubmed:21348467]
J Nat Prod. 2011 Apr 25;74(4):644-9.
The first and efficient syntheses of the naturally occurring prenylated stilbenes Chiricanine A (2), arahypin-1 (3), trans-arachidin-2 (4), trans-arachidin-3 (5), and arahypin-5 (6) are described. Syntheses of 2 and 3 were accomplished by either a convergent sequence or a one-step reaction starting from pinosylvin. Syntheses of 4, 5, and 6 were achieved from (E)-3,5-bis-methoxymethyl-4'-triisopropylsilyloxystilbene obtained by a Horner-Wadsworth-Emmons reaction between a benzaldehyde possessing bis-methoxymethyl ether groups and a benzyl phosphonate with a triisopropylsilyloxy group.


